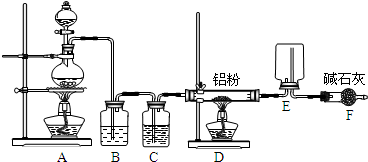
��Ŀ����
��8�֣�ijѧϰС������ͼװ�òⶨ��þ�Ͻ������������������������ԭ������.

����ʾ��þ���������ᷴӦ�������ܺͼӦ��2Al+2NaOH+2H2O=2NaAlO2+3H2����
ʵ��ǰ���Ƚ���þ�Ͻ���ϡ���н���Ƭ�̣���Ŀ���dz�ȥ��þ�Ͻ���������Ĥ��
��1��A���Լ�Ϊ .
���NaOH��Һ����ϡ���ᡱ��
��2����������ԣ���ҩƷ��ˮװ��������У����Ӻ�װ�ú�����еIJ������У��ټ�¼
C��Һ��λ�ã��ڽ�B��ʣ�������ˣ�ϴ�ӣ�������أ��۴�B�в���������������ָ������£�����A��B�еμ������Լ����ݼ�������ԣ�����������ʹD��C��Һ����ƽ��
����������˳���� ��������ţ�
��3��B�з�����Ӧ�����ӷ���ʽΪ .
��4��ʵ������У���δϴ�ӹ������õIJ���������������������� .
���ƫ����ƫС����������Ӱ�족��
��5����ʵ������þ�Ͻ������Ϊa g,����������Ϊb ml���ѻ���Ϊ��״������B��ʣ����������Ϊc g�����������ԭ������Ϊ .���ú�a��b��c�Ĵ���ʽ��ʾ��
��8�֣�
��1��NaOH��Һ.��1�֣�
��2���٢ܢۢޢ٢ڣ�2�֣���
��3��2Al+2OH-+2H2O=2AlO2�� +3H2����2�֣�
��4��ƫС. ��1�֣�
��5��33600��a-c��/b (2��)
����������