��Ŀ����
��10�֣���1���ڶ���ʵ���У��������в����ᵼ��ʵ����ƫ�͵��� ������ţ���
A���к��Ȳⶨʵ�����Ի�����˿��������滷�β��������
B������100mL 2 mol/L��NaCl��Һ���Խ�ͷ�ιܼ�ˮ����ʱ��Һ���Գ�������ƿ�̶��ߣ�����ҡ�Ⱥ�Һ����͵�ǡ����̶�������
C���ⶨ����ͭ����ᾧˮ���������Ȳ�����ʱ�����������彦����
D����ȡһ��������ˮ̼���Ʒ�ĩ����ϡ����ζ����ü�����ָʾ�������ⶨ����Ũ�ȡ���ʽ�ζ���������ˮ��ϴ��δ�ô�װҺ��ϴ������װҺ���ζ���
��2������ʵ�������ʵ�����ó��Ľ���һ����ȷ���� ������ţ�[��Դ:ZXXK]
A������100mL 1��00mol/L��NaCl ��Һʱ������������ƽȷ��ȡ5��85g NaCl
����
B��ij��ɫ��Һ�м������������ɫ��ζ��������ʹ����ʯ��ˮ����ǵ����壬��
����Һ��һ�����д�����HCO3-
C���Ʊ�Fe(OH)3����ʱ��Ӧ����ˮ�мӱ��͵�FeCl3 ��Һ�����������ȵ���Һ��
���ɫΪֹ
D����ȥ�������л��е�����NaCl����AgNO3��Һ�����
E������ѧ������ͭ������Ľᾧˮ�����IJⶨ��ʵ���У�������Ҫ�����Ĵγ�����
��
F���ⶨ�к���ʵ���У�ÿ��ʵ���Ӧ���������¶ȣ���������ʼ�¶ȣ�NaOH��Һ
����ʼ�¶Ⱥͷ�Ӧ����Һ������¶�
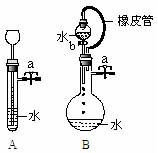
��3��������ͼ�����ش��������⣺

�ٹر�ͼAװ���е�ֹˮ��a�ӳ���©�����Թ���ע��һ������ˮ�����ú���ͼ��ʾ�����жϣ�Aװ���Ƿ�©�������©����������©��������ȷ������
�ڹر�ͼBװ���е�ֹˮ��a��������b��ˮ�������µΣ�ֱ��ȫ��������ƿ�����жϣ�Bװ���Ƿ�©����
���©����������©��������ȷ������ ���ж������� ��
��1��ABD��2��CEF ��3���ٲ�©�� �ڲ���ȷ��������װ���Ƿ�©������ƿ����ѹ��©���Ϸ�����ѹ��ȣ�ˮ�����������¿���ȫ���롣
��������

��1���ڶ���ʵ���У��������в����ᵼ��ʵ����ƫ�͵��� ������ţ���
A���к��Ȳⶨʵ�����Ի�����˿��������滷�β��������
B������100mL 2 mol/L��NaCl��Һ���Խ�ͷ�ιܼ�ˮ����ʱ��Һ���Գ�������ƿ�̶��ߣ�����ҡ�Ⱥ�Һ����͵�ǡ����̶�������
C���ⶨ����ͭ����ᾧˮ���������Ȳ�����ʱ�����������彦����
D����ȡһ��������ˮ̼���Ʒ�ĩ����ϡ����ζ����ü�����ָʾ�������ⶨ����Ũ�ȡ���ʽ�ζ���������ˮ��ϴ��δ�ô�װҺ��ϴ������װҺ���ζ���
��2������ʵ�������ʵ�����ó��Ľ���һ����ȷ���� ������ţ�
A������100mL 1��00mol/L��NaCl ��Һʱ������������ƽȷ��ȡ5��85g NaCl
����
B��ij��ɫ��Һ�м������������ɫ��ζ��������ʹ����ʯ��ˮ����ǵ����壬��
����Һ��һ�����д�����HCO3-
C���Ʊ�Fe(OH)3����ʱ��Ӧ����ˮ�мӱ��͵�FeCl3 ��Һ�����������ȵ���Һ��
���ɫΪֹ
D����ȥ�������л��е�����NaCl����AgNO3��Һ�����
E������ѧ������ͭ������Ľᾧˮ�����IJⶨ��ʵ���У�������Ҫ�����Ĵγ�����
��
F���ⶨ�к���ʵ���У�ÿ��ʵ���Ӧ���������¶ȣ���������ʼ�¶ȣ�NaOH��Һ
����ʼ�¶Ⱥͷ�Ӧ����Һ������¶�
��3��������ͼ�����ش��������⣺
 |
�ٹر�ͼAװ���е�ֹˮ��a�ӳ���©�����Թ���ע��һ������ˮ�����ú���ͼ��ʾ�����жϣ�Aװ���Ƿ�©�������©����������©��������ȷ������
�ڹر�ͼBװ���е�ֹˮ��a��������b��ˮ�������µΣ�ֱ��ȫ��������ƿ�����жϣ�Bװ���Ƿ�©����
���©����������©��������ȷ������ ���ж������� ��
��1���ڶ���ʵ���У��������в����ᵼ��ʵ����ƫ�͵��� ������ţ���
A���к��Ȳⶨʵ�����Ի�����˿��������滷�β��������
B������100mL 2 mol/L��NaCl��Һ���Խ�ͷ�ιܼ�ˮ����ʱ��Һ���Գ�������ƿ�̶��ߣ�����ҡ�Ⱥ�Һ����͵�ǡ����̶�������
C���ⶨ����ͭ����ᾧˮ���������Ȳ�����ʱ�����������彦����
D����ȡһ��������ˮ̼���Ʒ�ĩ����ϡ����ζ����ü�����ָʾ�������ⶨ����Ũ�ȡ���ʽ�ζ���������ˮ��ϴ��δ�ô�װҺ��ϴ������װҺ���ζ���
��2������ʵ�������ʵ�����ó��Ľ���һ����ȷ���� ������ţ�
A������100mL 1��00mol/L��NaCl ��Һʱ������������ƽȷ��ȡ5��85g NaCl
����
B��ij��ɫ��Һ�м������������ɫ��ζ��������ʹ����ʯ��ˮ����ǵ����壬��
����Һ��һ�����д�����HCO3-
C���Ʊ�Fe(OH)3����ʱ��Ӧ����ˮ�мӱ��͵�FeCl3 ��Һ�����������ȵ���Һ��
���ɫΪֹ
D����ȥ�������л��е�����NaCl����AgNO3��Һ�����
E������ѧ������ͭ������Ľᾧˮ�����IJⶨ��ʵ���У�������Ҫ�����Ĵγ�����
��
F���ⶨ�к���ʵ���У�ÿ��ʵ���Ӧ���������¶ȣ���������ʼ�¶ȣ�NaOH��Һ
����ʼ�¶Ⱥͷ�Ӧ����Һ������¶�
��3��������ͼ�����ش��������⣺
 |
�ٹر�ͼAװ���е�ֹˮ��a�ӳ���©�����Թ���ע��һ������ˮ�����ú���ͼ��ʾ�����жϣ�Aװ���Ƿ�©�������©����������©��������ȷ������
�ڹر�ͼBװ���е�ֹˮ��a��������b��ˮ�������µΣ�ֱ��ȫ��������ƿ�����жϣ�Bװ���Ƿ�©����
���©����������©��������ȷ������ ���ж������� ��
 mol/L��NaCl��Һ���Խ�ͷ�ιܼ�ˮ����ʱ��Һ���Գ�������ƿ�̶��ߣ�����ҡ�Ⱥ�Һ����͵�ǡ����̶�������
mol/L��NaCl��Һ���Խ�ͷ�ιܼ�ˮ����ʱ��Һ���Գ�������ƿ�̶��ߣ�����ҡ�Ⱥ�Һ����͵�ǡ����̶�������