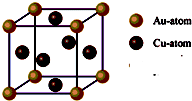
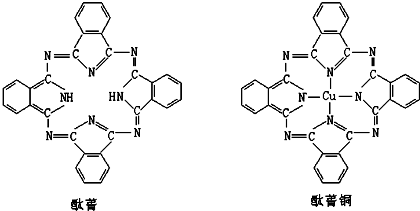
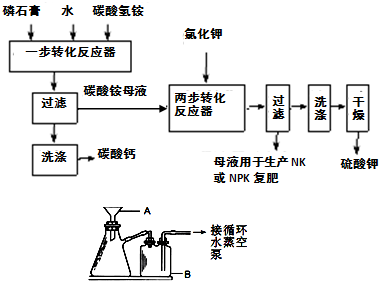
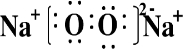��Ŀ����
��10�֣���������������Ҫ�Ľ��������ǵĵ��ʼ����������Ÿ��Ե����ʡ�
(1)��һ���¶��£���������������һ����̼�������з�Ӧ��
Fe2O3��s����3CO��g�� 2Fe��s����3CO2��g����H��0
2Fe��s����3CO2��g����H��0
�ٸ÷�Ӧ��ƽ�ⳣ������ʽΪ��K=
�ڸ��¶��£���2Lʢ�� ��ĩ���ܱ�������ͨ��CO���壬10min�������˵�����11��2g����10min��CO��ƽ����Ӧ����Ϊ
��ĩ���ܱ�������ͨ��CO���壬10min�������˵�����11��2g����10min��CO��ƽ����Ӧ����Ϊ
(2)ijЩ�����������ĩ��Al����þ������ȼ�¿��Է������ȷ�Ӧ�����з�Ӧ����(v)
���¶�(T)�Ĺ�ϵʾ��ͼ�������ȷ�Ӧ��ӽ����� ��
(3)Fe3+�κ�Al3+�����������кܶ����Ƶĵط���������������ܵĽ�״�����������ھ�ˮ��Ҳ�в�֮ͬ������Fe3+�������������л�ԭ�ԣ�Al3+ֻ�������ԡ���Fe3+ֻ���ڼ��Խ����в��ܱ�����ΪFeO42-����������з���ʽ��
Fe(OH)3 + ClO- + == FeO42- + Cl- + ;
(4)����ag Fe��Al�Ļ��������2mol/L�������������У�������Һ�м���������6mol/L��NaOH��Һ����ַ�Ӧ�����ˣ�ϴ�ӣ��������գ��������ù����������Ϊag����ԭ�������Al����������Ϊ
��1����c3(CO2)/c3(CO) ; ��0.015mol��L -1��min-1
-1��min-1
��2��b
��3�� 2 Fe(OH)3 + 3 ClO- + 4 OH- == 2 FeO42- + 3 Cl- + 5 H2O ;
��4��30%
����

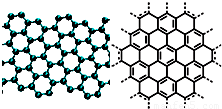
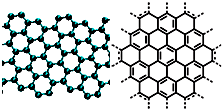 A����������ѧ����־������2009��ʮ���ѧͻ��֮һ��ʯīϩ���о���Ӧ�÷����ͻ�ƣ�ʯīϩ����ԭ�Ӽ��ĺ�ȡ�����ĵ�ѧ���ܡ���ɫ�Ļ�ѧ�ȶ��Ժ�����ѧ�ȶ��ԣ��Ʊ�ʯīϩ������ʯī���뷨����ѧ����������ȣ�ʯīϩ�����ģ�ͼ����ӽṹʾ��ͼ���ң�
A����������ѧ����־������2009��ʮ���ѧͻ��֮һ��ʯīϩ���о���Ӧ�÷����ͻ�ƣ�ʯīϩ����ԭ�Ӽ��ĺ�ȡ�����ĵ�ѧ���ܡ���ɫ�Ļ�ѧ�ȶ��Ժ�����ѧ�ȶ��ԣ��Ʊ�ʯīϩ������ʯī���뷨����ѧ����������ȣ�ʯīϩ�����ģ�ͼ����ӽṹʾ��ͼ���ң�