��Ŀ����
a��ˮ������������CH3CH2OH(g)��H2O(g)��4H2(g)��2CO(g)����H����255.58 kJ��mol-1
b�����ִ�������CH3CH2OH(g)��1/2O2(g)��3H2(g)��2CO(g)����H����13.76 kJ��mol-1
������˵���������
B�����������ĵĽǶ�������b·�������������
C��a·����������Ҫ���ĺܶ�������������ʵ�����������岻��
D��b·�߷�Ӧ�����ɸ�������������ķ�Ӧ

ˮú������Ҫ
ȼ�Ϻͻ���ԭ�ϣ�����ˮ����ͨ�����ȵ�̿���Ƶã�
C (s) + H2O(g) ![]() CO (g) +H2 (g) ��H�� +131.3 kJ•mol��1
CO (g) +H2 (g) ��H�� +131.3 kJ•mol��1
��1��ʵ�ʹ�ҵ�����У���̿��������ͨ��ˮ�����Ϳ�������ԭ���� ��
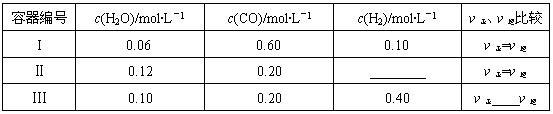
��2��һ���¶��£����������о�������������Ӧ����������̿�������������ʵ����ʵ���Ũ�ȼ����淴Ӧ���ʹ�ϵ���±���ʾ������д������Ӧ�Ŀո�
| ������� | c(H2O)/mol��L��1 | c(CO)/mol��L��1 | c(H2)/mol��L��1 | �����������Ƚ� |
| I | 0.06 | 0.60 | 0.10 | ����=���� |
| �� | 0.12 | 0.20 | ________ | ����=���� |
| �� | 0.10 | 0.20 | 0.40 | ����____���� |
��3�������Ҵ����ɵ��ۻ���ά�ص�������ԭ�Ϸ��ͻ�á������Ҵ��ɽ�����úϳ�����CO��H2����
���Ҵ������ϳ�������������·�ߣ�
a��ˮ������������CH3CH2OH(g)��H2O(g)��4H2(g)��2CO(g)
b�����ִ�������CH3CH2OH(g)��1/2O2(g)��3H2(g)��2CO(g)
ij���������о����������Ҵ��õ��ĺϳ����ϳ�һ���������͡��Ҵ�����һ�밴a��b��ʽ��Ӧ���ϳ����ϳ��������͵ķ�ӦΪ��2mCO��(2m��n)H2��2CmHn��2mH2O���ٶ��ϳɵ����������к���X��Y���ֳɷ֣���X��Y������8��̼ԭ�ӵ�����X�DZ���ͬϵ�Y��������
��X�ķ���ʽΪ ��Y�ķ���ʽΪ ��
��50����������Ϊ92%���Ҵ�������ת�����ٶ�����ת���ʾ�Ϊ100%���������տɻ��X������Ϊ���ٶ֣�

 CO (g) +H2 (g) ��H�� +131.3 kJ?mol��1����������������
CO (g) +H2 (g) ��H�� +131.3 kJ?mol��1����������������