��Ŀ����
 ��ͼ�dz�����һЩ���ʼ��仯����֮���ת����ϵͼ�����³�ѹ�£�D��F��K��Ϊ��ɫ�̼�����ζ�����壬CΪ���õĽ������ʣ�B���������ɫҺ�壬A���е���C��D��ȼ�����ɵĵ���ɫ���壬I�DZ��Ƹ�����õķ��ͷ۵���Ҫ�ɷ�֮һ������Ӧ�����ɵIJ�����������ȥ��
��ͼ�dz�����һЩ���ʼ��仯����֮���ת����ϵͼ�����³�ѹ�£�D��F��K��Ϊ��ɫ�̼�����ζ�����壬CΪ���õĽ������ʣ�B���������ɫҺ�壬A���е���C��D��ȼ�����ɵĵ���ɫ���壬I�DZ��Ƹ�����õķ��ͷ۵���Ҫ�ɷ�֮һ������Ӧ�����ɵIJ�����������ȥ����ش��������⣺
��1��K�ķ���ʽΪ
CO2
CO2
����2������A�ij�����;Ϊ
Ư��������
Ư��������
����дһ�ּ��ɣ�����3����Ӧ�ٵ����ӷ���ʽΪ
2Na+2H2O=2Na++2OH-+H2��
2Na+2H2O=2Na++2OH-+H2��
����Ӧ�ڵĻ�ѧ����ʽΪ2NaHCO3
Na2CO3+CO2��+H2O
| ||
2NaHCO3
Na2CO3+CO2��+H2O
��
| ||
������A���е���C��D��ȼ�����ɵĵ���ɫ���壬����ɫ����ΪNa2O2����AΪNa��CΪO2��DΪNa2O2��I�DZ��Ƹ�����õķ��ͷ۵���Ҫ�ɷ�֮һ��ӦΪNaHCO3��B���������ɫҺ�壬ӦΪH2O����EΪNaOH��FΪH2��KΪCO2��HΪNa2CO3��������ʵ����ʺ���ĿҪ��ɽ����⣮
����⣺A���е���C��D��ȼ�����ɵĵ���ɫ���壬����ɫ����ΪNa2O2����AΪNa��CΪO2��DΪNa2O2��I�DZ��Ƹ�����õķ��ͷ۵���Ҫ�ɷ�֮һ��ӦΪNaHCO3��B���������ɫҺ�壬ӦΪH2O����EΪNaOH��FΪH2��KΪCO2��HΪNa2CO3��
��1�������Ϸ�����֪KΪCO2���ʴ�Ϊ��CO2��
��2��AΪNa2O2������Ư���ԡ������ԣ�����ˮ�������̼��Ӧ���������������ڹ��������ʴ�Ϊ��Ư����������
��3����Ӧ��ΪNa��ˮ�ķ�Ӧ�����ӷ���ʽΪ2Na+2H2O=2Na++2OH-+H2����NaHCO3���ȶ������ȿɷֽ�����Na2CO3��CO2����Ӧ�ķ���ʽΪ2NaHCO3
Na2CO3+CO2��+H2O��
�ʴ�Ϊ��2Na+2H2O=2Na++2OH-+H2����2NaHCO3
Na2CO3+CO2��+H2O��
��1�������Ϸ�����֪KΪCO2���ʴ�Ϊ��CO2��
��2��AΪNa2O2������Ư���ԡ������ԣ�����ˮ�������̼��Ӧ���������������ڹ��������ʴ�Ϊ��Ư����������
��3����Ӧ��ΪNa��ˮ�ķ�Ӧ�����ӷ���ʽΪ2Na+2H2O=2Na++2OH-+H2����NaHCO3���ȶ������ȿɷֽ�����Na2CO3��CO2����Ӧ�ķ���ʽΪ2NaHCO3
| ||
�ʴ�Ϊ��2Na+2H2O=2Na++2OH-+H2����2NaHCO3
| ||
���������⿼��������ƶϣ���Ŀ�ѶȲ���������ʵ���ɫΪ�ƶϵ�ͻ�ƿڣ�ע�����������ʵ����ʣ�

��ϰ��ϵ�д�
�����Ŀ


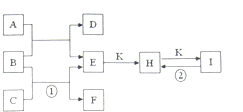 ��ͼ�dz�����һЩ���ʼ��仯����֮���ת����ϵͼ�����³�ѹ�£�D��F��K��Ϊ��ɫ�̼�����ζ�����壬CΪ���õĽ������ʣ�B���������ɫҺ�壬A���е���C��D��ȼ�����ɵĵ���ɫ���壬I�DZ��Ƹ�����õķ��ͷ۵���Ҫ�ɷ�֮һ������Ӧ�����ɵIJ�����������ȥ��
��ͼ�dz�����һЩ���ʼ��仯����֮���ת����ϵͼ�����³�ѹ�£�D��F��K��Ϊ��ɫ�̼�����ζ�����壬CΪ���õĽ������ʣ�B���������ɫҺ�壬A���е���C��D��ȼ�����ɵĵ���ɫ���壬I�DZ��Ƹ�����õķ��ͷ۵���Ҫ�ɷ�֮һ������Ӧ�����ɵIJ�����������ȥ��
