��Ŀ����
��18�֣���ҵ�����Ĵ����г���������NaCl���ʡ�Ϊ�ⶨij������Ʒ�Ĵ��ȣ���ѧ����С�����������ʵ�鷽����
������һ��ȡ��Ʒ�ܽ���Լ�ʹCO32���������ⶨ������������
������������0.100 mol/L������
������������ϡ�ὫCO32��ת��ΪCO2���ⶨCO2��������
���1-4С�⣺
1������һ�IJ��������У��ٳ������ܽ���Ʒ���ڼ���������BaCl2��Һ���۹��ˣ���ϴ�ӣ��ݸ�����������к��ز���������ʱ���趨��ʵ������Ϊ____________________���ж��Ƿ�ﵽ���صı���_____________________________________________________��
2���������ľ������Ϊ��

�ٲ���1����Ҫ��������_____________________________________________��
�ڵζ�ʱѡ���ָʾ��Ϊ���ȡ�����Һ____________________________________ʱ��˵���ﵽ�˵ζ��յ㡣
��Ϊ����żȻ��ͨ���Ĵ����취��_______________________________��
�ܵζ�ʱ����ƿ��������Һ�彦������ⶨ���_________����ѡ�ƫ�ߡ�����ƫ�͡�������Ӱ�족����ͬ������ʢװδ֪Һ����ƿ������ˮϴ����δ��δ֪Һ��ϴ���ⶨ���_________��
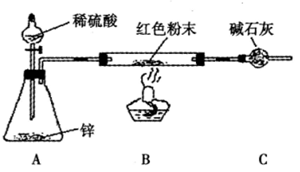
3����������ʵ��װ������ͼ��

���������У��ټ��װ�õ������ԣ����ڸ������װ����ʯ�ң���������ΪW1 g���۳���W2 g��Ʒװ����ƿB�У��ܹر�ֹˮ�У��ݻ�������ϡH2SO4�����ٲ�������Ϊֹ���� ��ֹˮ�У�����������������ӣ��ٳ�������ܣ�����ΪW3 g��
�÷�����Ʒ�д������������Ϊ__________________________���ô���ʽ��ʾ����
��ͼ��װ��A��������_____________________��װ��C��������_____________________����ͬѧ��Ϊ�����е�ˮ������������ܵ��²������_____________(�ƫ����ƫС������Ӱ�족�����Ľ���ʩ������______________________________________________��
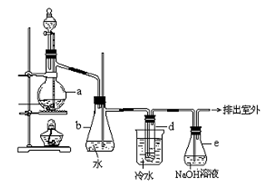
4��������ͼװ�ö���Ʒ���з�����������ƿ���ռ�����Һ��������������Ʒ��̼���Ƶĺ�����������װ���У����������______����ѡ����ţ�


A B


C D
������һ��ȡ��Ʒ�ܽ���Լ�ʹCO32���������ⶨ������������
������������0.100 mol/L������
������������ϡ�ὫCO32��ת��ΪCO2���ⶨCO2��������
���1-4С�⣺
1������һ�IJ��������У��ٳ������ܽ���Ʒ���ڼ���������BaCl2��Һ���۹��ˣ���ϴ�ӣ��ݸ�����������к��ز���������ʱ���趨��ʵ������Ϊ____________________���ж��Ƿ�ﵽ���صı���_____________________________________________________��
2���������ľ������Ϊ��

�ٲ���1����Ҫ��������_____________________________________________��
�ڵζ�ʱѡ���ָʾ��Ϊ���ȡ�����Һ____________________________________ʱ��˵���ﵽ�˵ζ��յ㡣
��Ϊ����żȻ��ͨ���Ĵ����취��_______________________________��
�ܵζ�ʱ����ƿ��������Һ�彦������ⶨ���_________����ѡ�ƫ�ߡ�����ƫ�͡�������Ӱ�족����ͬ������ʢװδ֪Һ����ƿ������ˮϴ����δ��δ֪Һ��ϴ���ⶨ���_________��
3����������ʵ��װ������ͼ��

���������У��ټ��װ�õ������ԣ����ڸ������װ����ʯ�ң���������ΪW1 g���۳���W2 g��Ʒװ����ƿB�У��ܹر�ֹˮ�У��ݻ�������ϡH2SO4�����ٲ�������Ϊֹ���� ��ֹˮ�У�����������������ӣ��ٳ�������ܣ�����ΪW3 g��
�÷�����Ʒ�д������������Ϊ__________________________���ô���ʽ��ʾ����
��ͼ��װ��A��������_____________________��װ��C��������_____________________����ͬѧ��Ϊ�����е�ˮ������������ܵ��²������_____________(�ƫ����ƫС������Ӱ�족�����Ľ���ʩ������______________________________________________��
4��������ͼװ�ö���Ʒ���з�����������ƿ���ռ�����Һ��������������Ʒ��̼���Ƶĺ�����������װ���У����������______����ѡ����ţ�


A B


C D
1.������ƽ��ǰ�����γ��������������0.001g��
2. ���ձ�����������100mL����ƿ����ͷ�ι�
����Һ�ɻ�ɫǡ�ñ�Ϊ��ɫ���Ұ�����ڲ���ɫ
����2~3��ƽ��ʵ��
��ƫ�ͣ���Ӱ��
3. �����տ����е�CO2������CO2�е�ˮ����������CO2���壩��ƫ���ڸ�����ұ��ټ�һ��װ�м�ʯ�ҵĸ����
�����տ����е�CO2������CO2�е�ˮ����������CO2���壩��ƫ���ڸ�����ұ��ټ�һ��װ�м�ʯ�ҵĸ����
4.B
2. ���ձ�����������100mL����ƿ����ͷ�ι�
����Һ�ɻ�ɫǡ�ñ�Ϊ��ɫ���Ұ�����ڲ���ɫ
����2~3��ƽ��ʵ��
��ƫ�ͣ���Ӱ��
3.
 �����տ����е�CO2������CO2�е�ˮ����������CO2���壩��ƫ���ڸ�����ұ��ټ�һ��װ�м�ʯ�ҵĸ����
�����տ����е�CO2������CO2�е�ˮ����������CO2���壩��ƫ���ڸ�����ұ��ټ�һ��װ�м�ʯ�ҵĸ����4.B
��

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ