��Ŀ����
2��ij����M��һ����������������Ϊȷ���仯ѧʽ��ijС����Ʋ����������ʵ�飺
��֪��
������M���������Ӻ�һ�ֺ��������ɣ��仯ѧʽ�е�ԭ�Ӹ�����Ϊ1��2��4��
����ͼ�У���1.66g����������ˮ���μ�����ϡ������ټ���1.12g��ԭ���ۣ�ǡ����ȫ��Ӧ�û����ҺN��
�۸�С��ͬѧ����ҺN��Ϊ���ȷݣ��ֱ�·�ߢ�·�ߢ����ʵ�飻
����·�ߢ��У���������ҺN�еμ�����NaOH��Ԫ��X�պó�����ȫ�����˺����ڿ����г�����յô�����Fe2O3��ĩ1.20g���ٽ���Һ��һ�����������ɣ�ֻ�õ�2.84g�����IJ����ᾧˮ������W��
�밴Ҫ��ش��������⣺
����·�ߢ�������֪����ҺN�к��е���������Fe2+��
����ʵ������ͼ���Ƶã���������W�Ļ�ѧʽ��Na2SO4��
������M����Һ��1.12g��ԭ����ǡ����ȫ��Ӧ������ҺN�����ӷ�Ӧ����Ϊ2Fe+FeO42-+8H+�T3Fe2++4H2O��
���� 1.2g�����������ʵ���Ϊ��$\frac{1.2g}{160g/mol}$=0.0075mol������ҺN�к�����Ԫ�ص����ʵ���Ϊ��0.0075mol��2��2=0.03mol������Ϊ��56g/mol��0.03mol=1.68g��1.12g����������M��һ������FeԪ�أ���ԭ������M����Ԫ�ص����ʵ���Ϊ0.03mol-0.02mol=0.01mol���������EΪNa2SO4��2.84g Na2SO4���ʵ���Ϊ0.02mol��������W�к���Ԫ�أ�����WΪ����������ҷ����е�ԭ�Ӹ�����Ϊ1��2��4���ɵ�M�Ļ�ѧʽΪK2FeO4��
��·�ߢ�Ϊ�����������ӵķ�����
��K2FeO4��ϡ���ᡢ���۷�Ӧ������������������غ�ˮ�����������غ��֪WΪ����أ�
�۸���K2FeO4��ϡ���ᡢ���۷�Ӧ������������������غ�ˮд����Ӧ�Ļ�ѧ����ʽ��
��� �⣺�ٸ���·�ߢ��֪��N��Һ��һ�������������ӣ��ʴ�Ϊ��Fe2+��
��1.2g�����������ʵ���Ϊ��$\frac{1.2g}{160g/mol}$=0.0075mol������Һ�к�����Ԫ�ص����ʵ���Ϊ��0.0075mol��2��2=0.03mol������Ϊ��56g/mol��0.03mol=1.68g��1.12g����������M��һ������FeԪ�أ�����M�����е�ԭ�Ӹ�����Ϊ1��2��4����M�Ļ�ѧʽΪ��Na2FeO4���������̿�֪��Na2FeO4��ϡ���ᡢ��ԭ���۷�Ӧ������������������أ����������غ��֪WΪ����أ�
�ʴ�Ϊ��Na2SO4 ��
��1.66gNa2FeO4�����ʵ���Ϊ0.01mol��1.12g��ԭ���۵����ʵ���Ϊ0.02mol������Na2FeO4�뻹ԭ���۰����ʵ���֮��Ϊ1��2��Ӧ�����ӷ���ʽΪ��2Fe+FeO42-+8H+�T3Fe2++4H2O��
�ʴ�Ϊ��2Fe+FeO42-+8H+�T3Fe2++4H2O��
���� ���⿼����̽��������ɵķ�������Ŀ�ѶȽϴ������漰������ɵIJⶨ��Ũ��������ʡ����ӷ���ʽ����ѧ����ʽ����д�����ӵļ��鷽����֪ʶ������֪ʶ��϶࣬�ۺ��Խ�ǿ����ֿ�����ѧ�����Ӧ�û���֪ʶ��������

 �����������Ů��ͯ������ϵ�д�
�����������Ů��ͯ������ϵ�д�| A�� | NH4+��Ba2+��NO3-��CO32- | B�� | Fe2+��OH-��SO42-��MnO4- | ||
| C�� | Na+��Mg2+��NO3-��SO42- | D�� | Na+��Fe3+��Cl-��AlO2- |
| A�� | �ö�������Ư��ֽ����ë��˿����ñ��ȣ�����Ϊ��������Ļ�ԭ����ʹ��ɫ���ʱ���������ɫ | |
| B�� | �����������ں��������Ϊ��������Դ������Ϊ�������ƾ���ǿ������������������̼ ��ˮ�������� | |
| C�� | ���μ�أ����϶��NaCl��Na2C03����ʩ������ʯ�࣬�ɽ��������ļ��ԣ�����Ϊʯ������ Na2C03��Ӧ����CaC03���� | |
| D�� | �ڱ������Ĺ�¯�ڱڣ�װ��þ�Ͻ��п�飬�Լ��ٹ�¯�ĸ�ʴ������Ϊþ��п�������ã���������ʹ��¯��Ϊԭ��صĸ������������� |
| A�� | ���³�ѹ�£�1 L 1 mol•L-1��BaCl2��Һ����NA��Cl- | |
| B�� | 1 mol Fe����ȫ��������Fe3O4��ʧȥ8 NA������ | |
| C�� | ���³�ѹ�£�14 g��CO��N2������庬�е�ԭ����ΪNA | |
| D�� | ���³�ѹ�£�22.4 L����������þ�۳�ַ�Ӧ��ת�Ƶĵ�����Ϊ2NA |

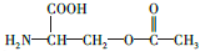 ��
�� b����ϡ������ˮ������������
b����ϡ������ˮ������������ ��
�� ��
��