��Ŀ����
��ҵ�ϵ�ⱥ��ʳ��ˮ����ȡ���ֻ���ԭ�ϣ����в���ԭ�Ͽ������Ʊ��ྦྷ�衣
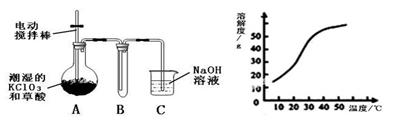
(1)��ͼ�����ӽ���Ĥ����ⱥ��ʳ��ˮʾ��ͼ����������������������________��NaOH��Һ�ij���Ϊ________(����ĸ)�����Ʊ���ʳ��ˮ�Ľ���Ϊ________(����ĸ)����������Ӧʹ�õ�Һ����________��
(2)�ྦྷ����Ҫ����SiHCl3��ԭ�����������丱����SiCl4���ۺ������ܵ��㷺��ע��
��SiCl4���������̿��(����ά��Ҫԭ����ͬ)������Ϊ������SiCl4��H2��O2��Ӧ�����������֣���ѧ����ʽΪ___________________________________��
��SiCl4��ת��ΪSiHCl3��ѭ��ʹ�ã�һ�������£���20 L�����ܱ������еķ�Ӧ��
3SiCl4(g)��2H2(g)��Si(s) 4SiHCl3(g)
4SiHCl3(g)
��ƽ���H2��SiHCl3���ʵ���Ũ�ȷֱ�Ϊ0.140 mol/L��0.020 mol/L����H2ȫ����Դ�����ӽ���Ĥ���ĵ���������������Ĵ�NaCl������Ϊ________kg��
(3)������Ĥ���۵�ⱥ��ʳ��ˮ������ȡ�����ƣ�ͬʱ�������������Ƶ�������213.0 kg������������________m3(��״��)��
(1)������a��d��Ũ���ᡡ(2)��SiCl4��2H2��O2 SiO2��4HCl����0.35��(3)134.4
SiO2��4HCl����0.35��(3)134.4
����

 ����ȫ���ִʾ��ƪ��ϵ�д�
����ȫ���ִʾ��ƪ��ϵ�д� �����߿����ϵ�д�
�����߿����ϵ�д� �㾦�½̲�ȫ�ܽ��ϵ�д�
�㾦�½̲�ȫ�ܽ��ϵ�д����յķϾ�п�̸ɵ�ؾ���������õ��̷�(��MnO2��Mn(OH)2��Fe����Ȳ�ͺ�̿��)�����̷���ȡMnO2�IJ�������ͼ��ʾ��
������ͼ��ʾ���貢�ο��������ݣ��ش��������⡣
| �� �� | ��ʼ���� | ������ȫ |
| Fe(OH)3 | 2.7 | 3.7 |
| Fe(OH)2 | 7.6 | 9.6 |
| Mn(OH)2 | 8.3 | 9.8 |
��1���ڼ�����������Ũ�����ȡ�̷ۣ�������Һ�к���Mn2+��Fe2+�ȡ�MnO2��Ũ���ᷴӦ�����ӷ��̷���ʽ�� _��
��2�����ʱ������ʱ����̽����ʵ�Ӱ������ͼ��ʾ����ҵ���õ��ǽ�ȡ60 min�������ԭ���� ��

��3���̷۾�Ũ�����ȡ������I��ȥ�������ʺ�����Һ�м�������H2O2��Һ���������� ��
��4������I������Һ�������������NaOH��Һ����pHԼΪ5.1����Ŀ���� ��
��5�����ˢ�������Һ��������H2O2��Һ������NaOH��Һ����pHԼΪ9��ʹMn2+ �����õ�MnO2����Ӧ�����ڷ���ʽΪ ��
��6����ҵ������KOH��MnO2Ϊԭ����ȡKMnO4����Ҫ�������̷��������У���һ����MnO2����KOH���飬��Ͼ��ȣ��ڿ����м������ۻ�����������������ȡK2MnO4���ڶ���Ϊ���K2MnO4��Ũ��Һ��ȡKMnO4��
�� ��һ����Ӧ�Ļ�ѧ����ʽΪ ��
�� ���K2MnO4��Ũ��Һʱ��������������ʵ������Ϊ ��