��Ŀ����
Ϊȷ��ij���ȼ���������������������ɣ��ֱ��������ʵ�顣
��1����ȡa g��Ʒ�������м���������NaOH��Һ��������ɵ����壨��״������ͬ�����Ϊb L����Ӧ�Ļ�ѧ����ʽ��__________________________����Ʒ������������_____________g��
��2����ȡa g��Ʒ�����ȼ��ǡ����ȫ��Ӧ���÷�Ӧ�Ļ�ѧ����ʽ�ǣ�_____________����������������������_____________��
��3������2���з�Ӧ������ȴ�����������ᣬ������ɵ��������Ϊc L���������루1������������������c��b��_____________��
��1��2Al��2NaOH��6H2O��2Na[Al(OH)4]��3H2����9b/11.2
��2�� 2Al��Fe2O3![]() Al2O3��2Fe�� 80��27 ��3�� 2��3
Al2O3��2Fe�� 80��27 ��3�� 2��3
����:
��1����Ʒ�е�����������NaOH��Һ��Ӧ����������NaOH��Һ��Ӧ�������ǻ��������ƺ�������
2Al �� 2NaOH �� 6H2O �� 2Na[Al(OH)4] �� 3H2��
2��27g��54g 3��22.4L��67.2L
m(Al) b L
m(Al)��b L��67.2L��54g��9b/11.2 g
��2�������зdz�ǿ�Ļ�ԭ�ԣ��ڵ�ȼ�����¿��������������ҷ�Ӧ�����������������ʣ�2Al��Fe2O3![]() Al2O3��2Fe���������ϻ�ѧ����ʽ��������������������������Ϊ80��27��
Al2O3��2Fe���������ϻ�ѧ����ʽ��������������������������Ϊ80��27��
��3�������ȷ�Ӧ�ķ���ʽ�����������������ʵ�������ȵģ���������ķ�Ӧ�У����ļ�̬���ߵ�+3�ۣ����ļ�ֻ̬���ߵ�+2�ۡ���ˣ�c��b��2��3��

 ѧ���쳵�����ּ��������ҵ�½����������ϵ�д�
ѧ���쳵�����ּ��������ҵ�½����������ϵ�д� �����ѧСѧ�꼶�νӵ������㽭��ѧ������ϵ�д�
�����ѧСѧ�꼶�νӵ������㽭��ѧ������ϵ�д� Сѧ�����ҵ���ϴ�ѧ������ϵ�д�
Сѧ�����ҵ���ϴ�ѧ������ϵ�д� ���Ž�����ٰθ��νӹ㶫���������ϵ�д�
���Ž�����ٰθ��νӹ㶫���������ϵ�д� �����������ҵ�������������ϵ�д�
�����������ҵ�������������ϵ�д�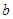 L����Ӧ�Ļ�ѧ����ʽ��_________________����Ʒ������������____________g��
L����Ӧ�Ļ�ѧ����ʽ��_________________����Ʒ������������____________g��