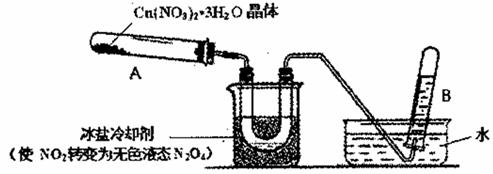
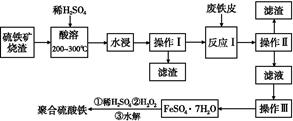
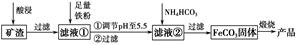
��Ŀ����
����ʯ��������õ�����[KAl(SO4)2��12H2O]���������Ʊ�Al��K2SO4����H2SO4�Ĺ��չ���������ʾ��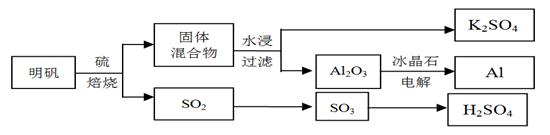
���������Ļ�ѧ����ʽΪ��4KAl(SO4)2��12H2O + 3S = 2K2SO4 + 2Al2O3 + 9SO2 + 48H2O
��ش��������⣺
��1���ڱ��������ķ�Ӧ�У���ԭ���� ��
��2����ˮ�������Һ�еõ�K2SO4����ķ����� ��
��3��Al2O3��һ�������¿��Ƶ�AlN���侧��ṹ��ͼ��ʾ���þ�����Al����λ���� ��
��4����Al��NiO(OH)Ϊ�缫��NaOH��ҺΪ���Һ���һ�����͵�أ��ŵ�ʱNiO(OH)ת��ΪNi(OH)2���õ�ط�Ӧ�Ļ�ѧ����ʽ�� ��
��5�����ղ�����SO2�����������ᡣ��֪25�桢101kPaʱ��
2SO2��g��+ O2��g�� 2SO3��g�� ��H1 = ��197 kJ /mol��
2SO3��g�� ��H1 = ��197 kJ /mol��
H2O��g�� H2O��l�� ��H2 = ��44 kJ/mol��
H2O��l�� ��H2 = ��44 kJ/mol��
2SO2��g��+ O2��g��+ 2H2O��g��=2H2SO4��aq�� ��H3 = ��545 kJ/mol��
��SO3��g����H2O��l����Ӧ���Ȼ�ѧ����ʽ�� ��
����948 t������M =" 474" g/mol������SO2��������Ϊ96%���ɲ�����������Ϊ98%������ t��
��1��S ��2�֣�
��2�������ᾧ��2�֣�
��3��4 ��2�֣�
��4��Al+3NiO(OH)+H2O==NaAlO2+3Ni(OH)2 ��3�֣�
��5��SO3(g)+H2O(l)==H2SO4(aq), ��H="-130" KJ/mol ��3�֣� ��432t ��3�֣�
�����������������������ԭ��Ӧ�����ʵķ�����ᴿ�����ʵĽṹ��֪ʶ���ںϵ����չ����У���������ԭ���ԭ�����Ȼ�ѧ����ʽ����д���Ѷ����С�
���㣺������ԭ��Ӧ��ԭ���ԭ�����Ȼ�ѧ����ʽ����д��

 ��1����Ԫ�¿�������ĩϵ�д�
��1����Ԫ�¿�������ĩϵ�д�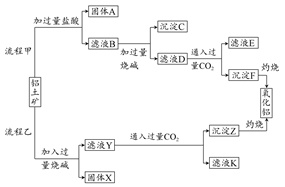
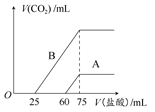

 ____CuAlO2��________����
____CuAlO2��________����