��Ŀ����
�о���ѧϰ��̽��ʵ�����о��õ�NaOH�ı��ʳ̶�[�о�����] �ȳ�ȡ13.3g��NaOH��Ʒ������ΪNa2CO3���������Һ��Ȼ������Һ����μ�����������Ϊ14.6%��ϡ���ᣬ��������CO2�������ⶨNa2CO3���������Ӷ���һ��ȷ����Ʒ��NaOH�ı��ʳ̶ȡ�
[�������] ʵ���ü���ϡ��������������CO2�����������ϵ����ͼ��ʾ��
|
Na2CO3������/g |
|
|
����NaOH������/g |
|
|
NaOH�ı��ʳ̶ȣ�������������ʾ�� |
|
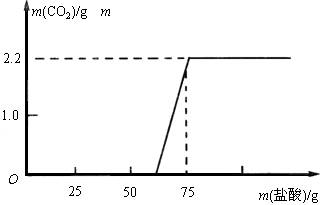
��д�ϱ���������������С�����һλ��
[����̽��] ��ʵ���������NaOH��Ӧ���������������
[��������] ���ݡ���NaOH��Ӧ���������������������ͼ���㷢����ʲô����?
������
[�������]
[����̽��] m((NaOH))=13.3g-5.3g=8g NaOH+HCl�TNaCl+H2O 40 36.5 8g m(HCl) m(HCl)=8g´ m[HCl(aq)]= [��������] NaOH���кͺμ����ᣬΪʲôû����������CO2����?������������Ҳ���֣�
|
��ʾ��
| ϡ�������Ⱥ��������Ʒ�Ӧ��û�г������ɣ����к���ȫ�ź�̼���Ʒ�Ӧ�ų�������̼���塣
|

��1����ͬѧ���α��Ļ���֪ʶ����������ͼ��������������������ݣ�
| ��ȡ���� | ����ҪƷ | װ������˳�� | ��Ӧ�Ļ�ѧ����ʽ |
| ���� | ����غͶ������� | ||
| ������̼����� | ����ʯ��ϡ���� |
K2ʱ������I����װ�ÿ�ֱ�ӽ��е�ʵ����
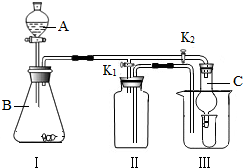
�ٴ���ʯ��ϡ���ᷴӦ��ȡ������̼
��п��ϡ���ᷴӦ��ȡ����
��3����ͬѧ��Ϊ�ڲ��ı�I����װ�õ�������
λ�õ�ǰ���£���װ�ÿ����ڹ������ƣ�Na2O2����ˮ��Ӧ��ȡ��������һ����Ϊ�������ƣ���÷�Ӧ�Ļ�ѧ����ʽΪ��
��4����ͬѧ��K2���ر�K1������I����װ������֤���ɵĶ�����̼�к���ˮ��������ʱC��ʢ�ŵ�������
��5����������غͶ������̵Ļ������ȡO2��Ĺ���������ٶ�����ȫ��Ӧ�������»��մ�����
��ͨ�������IJ�ʵ��������ն������̣���ȷ�������Ⱥ�˳����
a�����b���ܽ�c������d��ϴ��
���������Ȼ��ؾ�������50g��������Ϊ5%���Ȼ�����Һ����Ҫ�Ȼ��ص�����Ϊ
�۹��ˡ�����ʱ�����õ���������
A���ƾ���B���ձ�C��������D��©��E����Ͳ
��6��С����6.5g���ܺ���ͭ����������þ�е�һ�ֻ��ֽ������ʵ�п�ۣ���������ϡ������ȫ��Ӧʱ������0.2g���������п����һ�����еĽ���������
ij�о���ѧϰС���ͬѧ����������װ��̽����ɫֲ������������Ƿ���
CO2�����������Ƶ�̽���������£���ش����е��й����⣮

��1�����裺��ɫֲ���ں�����������CO2���������
��2����Ʒ�����ʹ��ɫֲ���ڱܹ�ĺڰ��������������ã�������������в��������壮
��3���������ϣ�����ɫֲ�������ù��̣�ˮ+������̼һ���л���+����
����ɫֲ��������ù��̣��л���+����һ��������̼+ˮ+����
��4��ʵ�飺
| �������� | ��� |
| �� ����װ�ð���ͼ ��ʾ���Ӻò�װ�뻯ѧ�Լ��� C�з�����ɫֲ�� | �� Aװ�õ������� �� Bװ�õ������� �� C�������ֲ������ԭ����________________
|
| �� ��A�ĵ��ܿڻ������� �������һ��ʱ�� | �� Aװ����Ӧ�۲쵽��������ʯ��ˮ����� ��Dװ����Ӧ�۲쵽��������ʯ��ˮ����� |
��5�����������ۣ����ж����̽��ʵ��________����ɹ������ɹ�������
��������о���ѧϰС��̽��ʵ�鲻�ɹ���ԭ���Ƕ��ġ������ʵ�������������ҳ����ܵ�һ��ԭ��________________________________��
ij�о���ѧϰС���ͬѧ����������װ��̽����ɫֲ������������Ƿ���
CO2�����������Ƶ�̽���������£���ش����е��й����⣮

��1�����裺��ɫֲ���ں�����������CO2���������
��2����Ʒ�����ʹ��ɫֲ���ڱܹ�ĺڰ��������������ã�������������в��������壮
��3���������ϣ�����ɫֲ�������ù��̣�ˮ+������̼һ���л���+����
����ɫֲ��������ù��̣��л���+����һ��������̼+ˮ+����
��4��ʵ�飺
| �������� | ��� |
| �� ����װ�ð���ͼ ��ʾ���Ӻò�װ�뻯ѧ�Լ���C�з�����ɫֲ�� | �� Aװ�õ������� �� Bװ�õ������� �� C�������ֲ������ԭ����________________
|
| �� ��A�ĵ��ܿڻ������� �������һ��ʱ�� | �� Aװ����Ӧ�۲쵽��������ʯ��ˮ����� �� Dװ����Ӧ�۲쵽��������ʯ��ˮ����� |
��5�����������ۣ����ж����̽��ʵ��________����ɹ������ɹ�������
��������о���ѧϰС��̽��ʵ�鲻�ɹ���ԭ���Ƕ��ġ������ʵ�������������ҳ����ܵ�һ��ԭ��________________________________��
 34��ij�о���ѧϰС��̽��CuSO4��Һ��NaOH��Һ�ķ�Ӧ���
34��ij�о���ѧϰС��̽��CuSO4��Һ��NaOH��Һ�ķ�Ӧ���