��Ŀ����
��ѡ��ҩƷ���±��Ƕ�����ҩƷ����ʵ��ļ�¼��
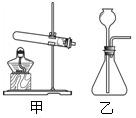
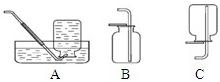
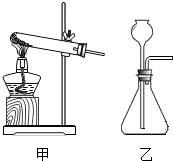
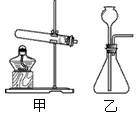
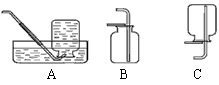
��ѡ��װ�á�ѡ�� ����װ�ã�����ţ���
����
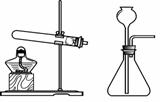
��B����ȼ�ŵ�ľ�����ڼ���ƿ�ڣ���ľ��Ϩ����֤������
�Ȳ���ȷ�������ɵ�����ͨ�����ʯ��ˮ�У���ʯ��ˮ����ǣ���������Ƕ�����̼

������ɶ�ʵ������ȡ������̼��ʵ��̽����
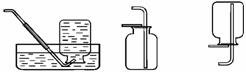
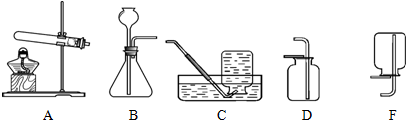
A B C D F
��ѡ��ҩƷ���±��Ƕ�����ҩƷ����ʵ��ļ�¼��
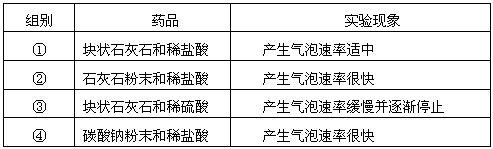
| ��� | ҩƷ | ʵ������ |
| �� | ��״ʯ��ʯ��ϡ���� | ���������������� |
| �� | ʯ��ʯ��ĩ��ϡ���� | �����������ʺܿ� |
| �� | ��״ʯ��ʯ��ϡ���� | �����������ʻ�������ֹͣ |
| �� | ̼���Ʒ�ĩ��ϡ���� | �����������ʺܿ� |
����ȡ���ռ��ĽǶȷ�����һ��ѡ��� ��ҩƷ������ĸ��ţ���ͬ��������ҩƷ������Ӧ�Ļ�ѧ����ʽΪ ��
��ѡ��װ�á�ѡ�� ����װ�á�
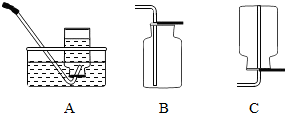
����ȡ���塣ѡ�� װ���ռ�������̼�������������� ��
��������顣�����ɵ�����ͨ����ɫʯ����Һ�У���Һ��죬ȷ���������Ƕ�����̼�����ּ��鷽���Ƿ���ȷ������ȷ��˵�����ɣ�������ȷ��д����ȷ�ļ��鷽���� ��