��Ŀ����
32����1�������С��һ�����ʵ������ȡ������̼ҩƷѡ���̽����
С��������ҩƷ�������о���ʵ���¼���£�
����ȡ��
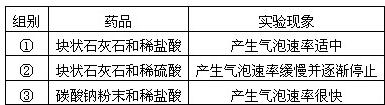
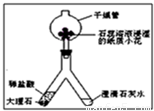
��2��������ͼ��ʾװ�ý��ж�����̼���Ʊ�������ʵ�飮
ʵ�����һ��ʱ����ˡ��ֹ��Ҷ˿��Թ۲쵽��������
С��������ҩƷ�������о���ʵ���¼���£�

����ȡ��
�ռ�
�ĽǶȷ�����һ��ѡ��ڢ���ҩƷ����������Ӧ�Ļ�ѧ����ʽΪCaCO3+2HCl=CaCl2+H2O+CO2��
����ѡ��ڢ���ҩƷ��ԭ������Ӧ����̫�죬�������ռ�
����2��������ͼ��ʾװ�ý��ж�����̼���Ʊ�������ʵ�飮

ʵ�����һ��ʱ����ˡ��ֹ��Ҷ˿��Թ۲쵽��������
����ʯ��ˮ�����
��������Ӧ�Ļ�ѧ����ʽΪCO2+Ca��OH��2�TCaCO3��+H2O
����ʯ����Һ��ʪ��ֽ��С������ɫ�仯Ϊ�ϱ��
������ȼ�ŵ�
��ľ���Ӹ���ܵ��Ϲܿ�������ڣ����Կ���ľ��Ϩ��
��˵��������̼������ȼ�գ�Ҳ��֧��ȼ��
�����ʣ�������ʵ������ȡ����ķ���װ��ѡ�������Ϊ��Ӧ���״̬�ͷ�Ӧ���������ռ�װ�õ�ѡ���������屾�����ܶȡ��ܽ��Ե��������ʣ����⣬��Ҫ���Ƿ�Ӧ�ٶȵȣ�������̼�����ʰ�������ȼ�գ�Ҳ��֧��ȼ�ա������ʯ��ˮ��Ӧ����ˮ��Ӧ��
����⣺��1��������̼����ȡҩƷ�ܶ࣬��ʵ�������п��Կ���������ҩƷ������������̼�����ǿ�״ʯ��ʯ��ϡ���ᷴӦһ���ֹͣ��̼���Ʒ�ĩ��ϡ���ᷴӦ�ٶ�̫�죬���ռ�����ˣ�ѡ���״ʯ��ʯ��ϡ��������ȡ������̼����Ӧԭ��ΪCaCO3+2HCl=CaCl2+H2O+CO2����
��2�����ˡ��ֹ���˵Ĵ���ʯ��ϡ���ᷴӦ����������̼��������̼��ʹ����ʯ��ˮ����ǣ���Ӧԭ��ΪCO2+Ca��OH��2�TCaCO3��+H2O����ˮ��Ӧ����̼�ᣬ���ɵ�̼��ʹ�ı���ɫʯ����Һ��ʪ��ֽ��С����ɺ�ɫ��������̼��ȼ�գ�Ҳ��֧��ȼ�գ������ʹȼ�ŵ�ľ��Ϩ��
�ʴ�Ϊ��
��1���ռ���CaCO3+2HCl=CaCl2+H2O+CO2������Ӧ����̫�죬�������ռ�
��2������ʯ��ˮ����ǣ�CO2+Ca��OH��2�TCaCO3��+H2O���ϱ�죻ȼ�ŵģ�ľ��Ϩ�𣻲�ȼ�գ�Ҳ��֧��ȼ��
��2�����ˡ��ֹ���˵Ĵ���ʯ��ϡ���ᷴӦ����������̼��������̼��ʹ����ʯ��ˮ����ǣ���Ӧԭ��ΪCO2+Ca��OH��2�TCaCO3��+H2O����ˮ��Ӧ����̼�ᣬ���ɵ�̼��ʹ�ı���ɫʯ����Һ��ʪ��ֽ��С����ɺ�ɫ��������̼��ȼ�գ�Ҳ��֧��ȼ�գ������ʹȼ�ŵ�ľ��Ϩ��
�ʴ�Ϊ��
��1���ռ���CaCO3+2HCl=CaCl2+H2O+CO2������Ӧ����̫�죬�������ռ�
��2������ʯ��ˮ����ǣ�CO2+Ca��OH��2�TCaCO3��+H2O���ϱ�죻ȼ�ŵģ�ľ��Ϩ�𣻲�ȼ�գ�Ҳ��֧��ȼ��
�����������ʵ�����Ʒ���Ҫ��ҩƷ����Ӧԭ��������װ�á��ռ�װ�á����顢������ʵ�����ע������ȷ�����бȽϡ����ɣ��ܽ�ʵ������ȡ�����һ��˼·�ͷ��������ʻ�ѧ���ʵ��о���ʵ�����֣�����ס�����ʵ�ʣ��������⡢������⣮

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
С����ʵ������ȡ������̼��ʵ����������̽����
��1�������С��һ�����ʵ������ȡ������̼ҩƷѡ���̽����
С��������ҩƷ�������о���ʵ���¼���£�
����ȡ���ռ��ĽǶȷ�����һ��ѡ��ڢ���ҩƷ����������Ӧ�Ļ�ѧ����ʽΪ______����ѡ��ڢ���ҩƷ��ԭ����______��
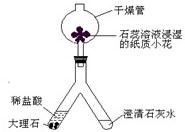
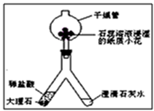
��2��������ͼ��ʾװ�ý��ж�����̼���Ʊ�������ʵ�飮ʵ�����һ��ʱ����ˡ��ֹ��Ҷ˿��Թ۲쵽��������______��������Ӧ�Ļ�ѧ����ʽΪ______����ʯ����Һ��ʪ��ֽ��С������ɫ�仯Ϊ______������______��ľ���Ӹ���ܵ��Ϲܿ�������ڣ����Կ���______��˵��������̼����______�����ʣ�
��3�����������ſ������������õ�������CO2��ͨ���������Ͽ�֪��25��ʱ��CO2��ˮ�Ͳ�������Һ�е��ܽ�������£�
���˸���CO2��ˮ���ܸ���Na2CO3��Ӧ����NaHCO3����NaHCO3����CO2��Ӧ��ԭ����������ű��Ͷ�NaHCO3��Һ�ķ����ռ�������CO2����������______��ԭ���ǣ�______������Ϊ�ռ�����CO2�Ͽ�ѧ�ķ�����______��
��1�������С��һ�����ʵ������ȡ������̼ҩƷѡ���̽����
С��������ҩƷ�������о���ʵ���¼���£�
| ��� | ҩƷ | ʵ������ |
| �� | ��״ʯ��ʯ��ϡ���� | ���������������� |
| �� | ��״ʯ��ʯ��ϡ���� | �����������ʻ�������ֹͣ |
| �� | ̼���Ʒ�ĩ��ϡ���� | �����������ʺܿ� |
��2��������ͼ��ʾװ�ý��ж�����̼���Ʊ�������ʵ�飮ʵ�����һ��ʱ����ˡ��ֹ��Ҷ˿��Թ۲쵽��������______��������Ӧ�Ļ�ѧ����ʽΪ______����ʯ����Һ��ʪ��ֽ��С������ɫ�仯Ϊ______������______��ľ���Ӹ���ܵ��Ϲܿ�������ڣ����Կ���______��˵��������̼����______�����ʣ�
| ����Һ������/100mL | �����յ�CO2���/mL |
| ����ˮ | 82.9 |
| ����NaCl��Һ | 58.6 |
| ����Na2SO4��Һ | 56 |
| ����NaHCO3��Һ | 120 |
���˸���CO2��ˮ���ܸ���Na2CO3��Ӧ����NaHCO3����NaHCO3����CO2��Ӧ��ԭ����������ű��Ͷ�NaHCO3��Һ�ķ����ռ�������CO2����������______��ԭ���ǣ�______������Ϊ�ռ�����CO2�Ͽ�ѧ�ķ�����______��

��1�������С��һ�����ʵ������ȡ������̼ҩƷѡ���̽����
С��������ҩƷ�������о���ʵ���¼���£�
����ȡ��______�ĽǶȷ�����һ��ѡ��ڢ���ҩƷ����������Ӧ�Ļ�ѧ����ʽΪ______����ѡ��ڢ���ҩƷ��ԭ����______��
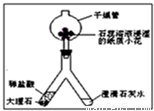
��2��������ͼ��ʾװ�ý��ж�����̼���Ʊ�������ʵ�飮
ʵ�����һ��ʱ����ˡ��ֹ��Ҷ˿��Թ۲쵽��������______��������Ӧ�Ļ�ѧ����ʽΪ______����ʯ����Һ��ʪ��ֽ��С������ɫ�仯Ϊ______������______��ľ���Ӹ���ܵ��Ϲܿ�������ڣ����Կ���______��˵��������̼����______�����ʣ�
С��������ҩƷ�������о���ʵ���¼���£�
| ��� | ҩƷ | ʵ������ |
| �� | ��״ʯ��ʯ��ϡ���� | ���������������� |
| �� | ��״ʯ��ʯ��ϡ���� | �����������ʻ�������ֹͣ |
| �� | ̼���Ʒ�ĩ��ϡ���� | �����������ʺܿ� |
��2��������ͼ��ʾװ�ý��ж�����̼���Ʊ�������ʵ�飮

ʵ�����һ��ʱ����ˡ��ֹ��Ҷ˿��Թ۲쵽��������______��������Ӧ�Ļ�ѧ����ʽΪ______����ʯ����Һ��ʪ��ֽ��С������ɫ�仯Ϊ______������______��ľ���Ӹ���ܵ��Ϲܿ�������ڣ����Կ���______��˵��������̼����______�����ʣ�
С����ʵ������ȡ������̼��ʵ����������̽����
��1�������С��һ�����ʵ������ȡ������̼ҩƷѡ���̽����
С��������ҩƷ�������о���ʵ���¼���£�
����ȡ���ռ��ĽǶȷ�����һ��ѡ��ڢ���ҩƷ����������Ӧ�Ļ�ѧ����ʽΪ______����ѡ��ڢ���ҩƷ��ԭ����______��
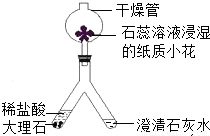
��2��������ͼ��ʾװ�ý��ж�����̼���Ʊ�������ʵ�飮ʵ�����һ��ʱ����ˡ��ֹ��Ҷ˿��Թ۲쵽��������______��������Ӧ�Ļ�ѧ����ʽΪ______����ʯ����Һ��ʪ��ֽ��С������ɫ�仯Ϊ______������______��ľ���Ӹ���ܵ��Ϲܿ�������ڣ����Կ���______��˵��������̼����______�����ʣ�
��3�����������ſ������������õ�������CO2��ͨ���������Ͽ�֪��25��ʱ��CO2��ˮ�Ͳ�������Һ�е��ܽ�������£�
���˸���CO2��ˮ���ܸ���Na2CO3��Ӧ����NaHCO3����NaHCO3����CO2��Ӧ��ԭ����������ű��Ͷ�NaHCO3��Һ�ķ����ռ�������CO2����������______��ԭ���ǣ�______������Ϊ�ռ�����CO2�Ͽ�ѧ�ķ�����______��
��1�������С��һ�����ʵ������ȡ������̼ҩƷѡ���̽����
С��������ҩƷ�������о���ʵ���¼���£�
| ��� | ҩƷ | ʵ������ |
| �� | ��״ʯ��ʯ��ϡ���� | ���������������� |
| �� | ��״ʯ��ʯ��ϡ���� | �����������ʻ�������ֹͣ |
| �� | ̼���Ʒ�ĩ��ϡ���� | �����������ʺܿ� |
��2��������ͼ��ʾװ�ý��ж�����̼���Ʊ�������ʵ�飮ʵ�����һ��ʱ����ˡ��ֹ��Ҷ˿��Թ۲쵽��������______��������Ӧ�Ļ�ѧ����ʽΪ______����ʯ����Һ��ʪ��ֽ��С������ɫ�仯Ϊ______������______��ľ���Ӹ���ܵ��Ϲܿ�������ڣ����Կ���______��˵��������̼����______�����ʣ�
| ����Һ������/100mL | �����յ�CO2���/mL |
| ����ˮ | 82.9 |
| ����NaCl��Һ | 58.6 |
| ����Na2SO4��Һ | 56 |
| ����NaHCO3��Һ | 120 |
���˸���CO2��ˮ���ܸ���Na2CO3��Ӧ����NaHCO3����NaHCO3����CO2��Ӧ��ԭ����������ű��Ͷ�NaHCO3��Һ�ķ����ռ�������CO2����������______��ԭ���ǣ�______������Ϊ�ռ�����CO2�Ͽ�ѧ�ķ�����______��

 С����ʵ������ȡ������̼��ʵ����������̽����
С����ʵ������ȡ������̼��ʵ����������̽����