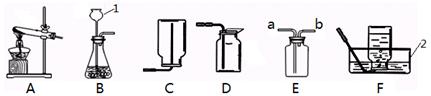
��Ŀ����
�������ͼ��ʾʵ��װ�ûش����⣺
��1��д��ͼ�б�����������ƣ�a�� ��b�� ��
��2������װ��B��ȡ������ҩƷѡ�ø�����أ�������Ӧ�Ļ�ѧ����ʽΪ �����Թܿڷ�һ������������ ��ʵ�鿪ʼǰ��ͼF��ʾ��������־��װ�����������õ������� ��
��3��ʵ��������װ��A��ȡ������̼���壬������Ӧ�Ļ�ѧ����ʽΪ ������4g CaCO3�μӷ�Ӧ�������� g CO2���壮ʵ�����ռ�������̼ѡ��װ�� ������ţ������ռ�ij����ֻ�ܲ���װ��E���ɴ��Ʋ��������е����������� ��
��1���ƾ��ƣ�����ƿ ��2��2KMnO4 K2MnO4+MnO2+O2��������KMnO4���뵼�ܣ����ܿ������� ��3��CaCO3+2HCl��CaCl2+H2O+CO2����1.76��D��������ˮ���ܶȱȿ���С
K2MnO4+MnO2+O2��������KMnO4���뵼�ܣ����ܿ������� ��3��CaCO3+2HCl��CaCl2+H2O+CO2����1.76��D��������ˮ���ܶȱȿ���С
���������������ͼ����֪�����������ƣ�������طֽ�����������ء��������̺�������������ֹ�������С�������뵼�ܣ�װ�����������ã��������Թ��¶����ߣ����������ݳ���������ݣ�ʵ������ȡ������̼ʹ�õ���̼��ƺ����ᷴӦ�����ݷ�Ӧ�Ļ�ѧ����ʽ����������ɶ�����̼�������������ռ�װ�õ�ѡ��ȡ����������ܶȺ��ܽ��ԣ��ݴ˽�ɡ�
��1����ͼ��֪��a�Ǿƾ��ƣ�b�Ǽ���ƿ������ƾ��ƣ�����ƿ��
��2��������������ֽܷ���������ء��������̺��������ڴ�װ���У��Թܿ�Ҫ��һ��������ֹ�������С���������뵼�ܣ��������Թܣ����Թ����¶����ߣ����������ݳ���װ�����������ã����������������2KMnO4 K2MnO4+MnO2+O2��������KMnO4���뵼�ܣ����ܿ������ݣ�
K2MnO4+MnO2+O2��������KMnO4���뵼�ܣ����ܿ������ݣ�
��3��ʵ������ȡ������̼ʹ�õ���̼��ƺ����ᣬ�����ܷ�Ӧ�����Ȼ��ơ�ˮ�Ͷ�����̼�������ɶ�����̼������Ϊx
CaCO3+2HCl��CaCl2+H2O+CO2��
100 44
4g x ��
��
���x��1.76g
������̼������ˮ������ʹ����ˮ���ռ���������̼���ܶȱȿ�����ʹ�������ſ������ռ���ֻ��ʹ��Eװ���ռ������壬��������ˮ���ܶȱȿ���С�����壬���CaCO3+2HCl��CaCl2+H2O+CO2����1.76��D��������ˮ���ܶȱȿ���С��
���㣺���鳣������ķ���װ�ú��ռ�װ����ѡȡ����

 �Ͻ�ƽСѧ��������ϵ�д�
�Ͻ�ƽСѧ��������ϵ�д� �Ƹ������������ϵ�д�
�Ƹ������������ϵ�д�