��Ŀ����
���������䡱�������͵��ǵ�������������Ҫ���ֽϽྻ�����廯ʯȼ�ϡ�����Ȼ��������ش�
��1����Ȼ������Ҫ�ɷ��� ���ѧʽ��
��2��ú����ʱ�ᷢ����˹��ը�¹ʣ���˹����Ҫ�ɷ�����Ȼ����ͬ��д����˹��ը�Ļ�ѧ����ʽ�� ������������
��3�����ݱ��������ҹ��Ϻ������������־�ġ���ȼ������������ȼ������CH4�� nH2O��������Ȼ����ˮ�ڸ�ѹ�����������γɵı�̬�������Ȼ�������̬���з�������������ַ�����
����һ���¶��£� ʹ����ӱ�̬���з��������
����һ��ѹǿ�£� ʹ����ӱ�̬���з��������
 |
��4����ʯȼ�ϵȲ���������Դ�������ݽߣ�������ʹ����ԴʱӦ������һҪ��Լ��Դ����Ҫ��һ����������������Դ���Ĺ۵㡣
�����и�ͼ�б�ʾ�ҹ����ܱ�־���ǣ� ��
A B C D
���д��������������õ���Դ��(����д����)
���û�ѧ����ʽ��ʾ��һ����ѧ��ת��Ϊ���ܵ����ӣ�
����ٳ�һ�������������е���ת���ɻ�ѧ�ܵ����ӣ�
��1��CH4��
(2) CH4+2O2![]() CO2+2H2O��
CO2+2H2O��
��3���ٽ���ѹǿ����ѹ�� �������¶ȣ������£���
��4���� C �� ��̫���ܡ����ܡ����ܡ����ܡ����ȡ���ϫ�ܵȣ���д�������ɸ��֣����� C+O2![]() CO2�����ȷ�Ӧ���ɸ��֣����ܵ�س�����ˮ�ȡ�
CO2�����ȷ�Ӧ���ɸ��֣����ܵ�س�����ˮ�ȡ�

��1���������ҹ�ij������һ�ݿ�������Ԥ������
| ָ �� | ����ʵ�� | ����Ԥ�� | ||
| API | ���������ȼ� | API | ���������ȼ� | |
| ������������ | 26 | �� | 20-35 | �� |
| �������� | 38 | �� | 30-45 | �� |
| �������� | 59 | �� | 55-75 | �� |
| ˵ �� | Ԥ�ƽ��տ��������Ϻã�����������Ӱ�죬������������ | |||
�ڶ�����������Ⱦ��������Ҫ�к�����֮һ����ȥ��������ķ����ж��֣�������һ�ַ����С�ʯ��ʯ������ԭ��Ϊʯ��ʯ����ƷΪʯ�ࣨCaSO4������д����صĻ�ѧ����ʽ��
��һ�����ֽ�ʯ��ʯ
�ڶ�������ʯ�����������Ļ���
������������������������Ӧ����ʯ��
��2��һ����̼Ҳ�dz����Ĵ�����Ⱦ������������������еIJ���ȫȼ�տɱ�ʾΪ
2C8H18+23O2��12CO2+4CO+18H2O
��ij���������ȤС���ͬѧ�����������IJ���������CO�����仯���ߣ��������ʾ����ʱ��0��24Сʱ���������ʾCO������������Ϊ�ȽϷ���ʵ�ʵ�����һ��ͼ
A��
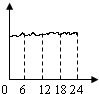 B��
B��
C��
 D��
D��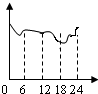
��Ŀǰ����������ȫ��ʹ�á��������䡱�������͵���Ȼ�������û��˹�ú������Ȼ���Ĺ�����һ��Ҫ��ԭ�е���߽��и�������������Ҳ���������ȫȼ�գ�����ʦ�ɼ���20.8g��Ȼ��ȼ�յIJ��ͨ����ˮ�Ȼ��ƹ��壨������������������10.8g����ԭ������һ����̼�Ͷ�����̼����������
��3����ý�屨����Ŀǰ�Ҿӻ����Ŀ�����Ⱦ��Ҫ��װ��ʱ���������к������ļ�ȩ��CH2O�����£����ڽӴ���ȩ����Ⱥ���ڡ��ǡ��������β������ķ����ʻ��������ӣ������ϳ��۵�һ�֡���ȩ���������к��еĹ������⣨H2O2��������Ч�ؽ���ȩ����Ϊ�����壬ͬʱ����ˮ����д���ù��̵Ļ�ѧ����ʽ��