��Ŀ����
д�����з�Ӧ�Ļ�ѧ����ʽ����գ�
��1����˿��������ȼ��
��2��þ����ϡ���ᷴӦ
��3��ʵ������˫��ˮ�Ͷ�������������
��4����Ȼ������Ҫ�ɷ�ΪCH4����ȫȼ��
��1����˿��������ȼ��
3Fe+2O2
Fe3O4
| ||
3Fe+2O2
Fe3O4
��
| ||
��2��þ����ϡ���ᷴӦ
Mg+2HCl=MgCl2+H2��
Mg+2HCl=MgCl2+H2��
���÷�Ӧ�ų�
�ų�
��������3��ʵ������˫��ˮ�Ͷ�������������
2H2O2
2H2O+O2��
| ||
2H2O2
2H2O+O2��
�����ж������̵�������
| ||
������
������
����4����Ȼ������Ҫ�ɷ�ΪCH4����ȫȼ��
CH4+2O2
CO2+2H2O
| ||
CH4+2O2
CO2+2H2O
��
| ||
���������ȸ��ݷ�Ӧԭ���ҳ���Ӧ��������Ӧ���������ݻ�ѧ����ʽ����д���������������д���ɣ�
����⣺��1������˿��������ȼ��������������������Ӧ�Ļ�ѧ����ʽΪ��3Fe+2O2
Fe3O4��
��2��þ����ϡ���ᷴӦ�����Ȼ�þ����������Ӧ�Ļ�ѧ����ʽΪ��Mg+2HCl=MgCl2+H2�����÷�Ӧ�ų��������ȣ�
��3�����������ڶ������̵Ĵ�����������ˮ����������Ӧ�Ļ�ѧ����ʽΪ��2H2O2
2H2O+O2����
��4����Ȼ������Ҫ�ɷ��Ǽ��飬�����ڵ�ȼ������ȼ�����ɶ�����̼��ˮ����Ӧ�Ļ�ѧ����ʽΪCH4+2O2
CO2+2H2O��
�ʴ�Ϊ����1��3Fe+2O2
Fe3O4����2��Mg+2HCl=MgCl2+H2�����ų�����3��2H2O2
2H2O+O2�����������4��CH4+2O2
CO2+2H2O��
| ||
��2��þ����ϡ���ᷴӦ�����Ȼ�þ����������Ӧ�Ļ�ѧ����ʽΪ��Mg+2HCl=MgCl2+H2�����÷�Ӧ�ų��������ȣ�
��3�����������ڶ������̵Ĵ�����������ˮ����������Ӧ�Ļ�ѧ����ʽΪ��2H2O2
| ||
��4����Ȼ������Ҫ�ɷ��Ǽ��飬�����ڵ�ȼ������ȼ�����ɶ�����̼��ˮ����Ӧ�Ļ�ѧ����ʽΪCH4+2O2
| ||
�ʴ�Ϊ����1��3Fe+2O2
| ||
| ||
| ||
�����������ѶȲ�����ѧ�����ݷ�Ӧԭ����д��ѧ����ʽ����������ѧ����ʽ��д�������ֵĴ����в����Ͽ���ʵ�������������غ㶨�ɡ���д������������ŵȣ�

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
��2009?¦�ף�С��ͬѧΪ�˽�һ������ԡ���Ļ�ѧ���ʡ������⣬������Э��������л��̽����
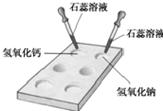
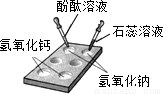
��1����ͼ��ʾ���ڰ�ɫ��ΰ��Ͻ���ʵ�飬�뽫ʵ�����������±���
��2��������������̼����ķ�Ӧ��д���÷�Ӧ�Ļ�ѧ�� ��ʽ______��
��3����������SO3�����������Ʒ�Ӧ������ķ�Ӧ���ƣ�д����һ��Ӧ�Ļ�ѧ����ʽ______��
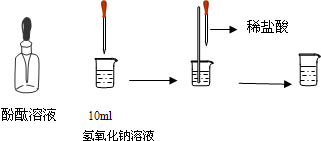
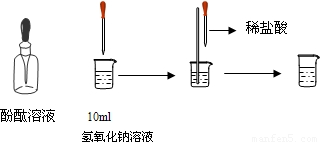
��4����ͼ��ʾ�����ձ��м���10mL����������Һ�����뼸�η�̪��Һ����Һ��______ɫ�����õι���������ϡ���ᣬ�����Ͻ�����Һ������Һ��ɫǡ�ñ����ɫΪֹ����һʵ��˵��������������������κ�ˮ����һ��Ӧ����______��Ӧ��
��5�����������ʵ������ۣ��Թ��ɳ��������ơ�������������Щ���ƵĻ�ѧ���ʣ�����д���㣩?
��______
��______��
��1����ͼ��ʾ���ڰ�ɫ��ΰ��Ͻ���ʵ�飬�뽫ʵ�����������±���
| ����������Һ | ����������Һ | |
| ����ɫʯ����Һ | ______ | ______ |
��3����������SO3�����������Ʒ�Ӧ������ķ�Ӧ���ƣ�д����һ��Ӧ�Ļ�ѧ����ʽ______��
��4����ͼ��ʾ�����ձ��м���10mL����������Һ�����뼸�η�̪��Һ����Һ��______ɫ�����õι���������ϡ���ᣬ�����Ͻ�����Һ������Һ��ɫǡ�ñ����ɫΪֹ����һʵ��˵��������������������κ�ˮ����һ��Ӧ����______��Ӧ��
��5�����������ʵ������ۣ��Թ��ɳ��������ơ�������������Щ���ƵĻ�ѧ���ʣ�����д���㣩?
��______
��______��