网址:http://m.1010jiajiao.com/timu3_id_85704[举报]
|
已知:①NH3(g)+HCl(g) ②NH3(g) ③HCl(g) ④NH3(aq)+HCl(aq) 则NH4Cl(s) | |
| [ ] | |
A. |
16.3 |
B. |
-16.3 |
C. |
335.7 |
D. |
-335.7 |
(16分)能量是一个世界性的话题,如何充分利用能量、开发新能源,为人类服务是广大科技工作者不懈努力的目标。
(1)如图所示,组成一个原电池.
①当电解质溶液为稀硫酸时:
Cu电极是_____(填“正”或“负”)极,其电极反应为____;
②当电解质溶液为浓硝酸时:
Cu电极是_____极,其电极反应为__________。
(2)请写出电解硫酸铜溶液的总化学方程式 。
(3)燃烧氢气时耗氧量小,放出热量多。已知4g H2燃烧生成液态水时放热为571.6kJ,试写出表示H2燃烧热的热化学方程式为: 。
(4)下图是一碳酸盐燃料电池(MCFC),以水煤气(CO、H2)为燃料,一定比例Li2CO3和Na2CO3低熔混合物为电解质。写出B极发生的电极反应式: 。
(5)请根据下面所给出的5个热化学方程式,判断反应④的反应热ΔH4是________。
①NH3(g)+HCl(g)===NH4Cl(s) ΔH1=-176 kJ·mol-1
②NH3(g)+H2O(l)===NH3·H2O(aq) ΔH2=-35.1 kJ·mol-1
③HCl(g)+H2O(l)===HCl(aq) ΔH3=-72.3kJ·mol-1
④NH4Cl(s)+H2O(l)===NH4Cl(aq) ΔH4=?
⑤NH3·H2O(aq)+HCl(aq)===NH4Cl(aq)+H2O(l) ΔH5=-52.3kJ·mol-1
查看习题详情和答案>>
(16分)能量是一个世界性的话题,如何充分利用能量、开发新能源,为人类服务是广大科技工作者不懈努力的目标。
(1)如图所示,组成一个原电池.

①当电解质溶液为稀硫酸时:
Cu电极是_____(填“正”或“负”)极,其电极反应为____;
②当电解质溶液为浓硝酸时:
Cu电极是_____极,其电极反应为__________。
(2)请写出电解硫酸铜溶液的总化学方程式 。
(3)燃烧氢气时耗氧量小,放出热量多。已知4g H2燃烧生成液态水时放热为571.6kJ,试写出表示H2燃烧热的热化学方程式为: 。
(4)下图是一碳酸盐燃料电池(MCFC),以水煤气(CO、H2)为燃料,一定比例Li2CO3和Na2CO3低熔混合物为电解质。写出B极发生的电极反应式: 。

(5)请根据下面所给出的5个热化学方程式,判断反应④的反应热ΔH4是________。
①NH3(g)+HCl(g)===NH4Cl(s) ΔH1=-176 kJ·mol-1
②NH3(g)+H2O(l)===NH3·H2O(aq) ΔH2=-35.1 kJ·mol-1
③HCl(g)+H2O(l)===HCl(aq) ΔH3=-72.3 kJ·mol-1
④NH4Cl(s)+H2O(l)===NH4Cl(aq) ΔH4=?
⑤NH3·H2O(aq)+HCl(aq)===NH4Cl(aq)+H2O(l) ΔH5=-52.3 kJ·mol-1
查看习题详情和答案>>
(16分)能量是一个世界性的话题,如何充分利用能量、开发新能源,为人类服务是广大科技工作者不懈努力的目标。
(1)如图所示,组成一个原电池.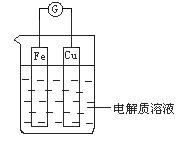
①当电解质溶液为稀硫酸时:
Cu电极是_____(填“正”或“负”)极,其电极反应为____;
②当电解质溶液为浓硝酸时:
Cu电极是_____极,其电极反应为__________。
(2)请写出电解硫酸铜溶液的总化学方程式 。
(3)燃烧氢气时耗氧量小,放出热量多。已知4g H2燃烧生成液态水时放热为571.6kJ,试写出表示H2燃烧热的热化学方程式为: 。
(4)下图是一碳酸盐燃料电池(MCFC),以水煤气(CO、H2)为燃料,一定比例Li2CO3和Na2CO3低熔混合物为电解质。写出B极发生的电极反应式: 。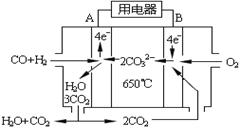
(5)请根据下面所给出的5个热化学方程式,判断反应④的反应热ΔH4是________。
①NH3(g)+HCl(g)===NH4Cl(s) ΔH1=-176 kJ·mol-1
②NH3(g)+H2O(l)===NH3·H2O(aq) ΔH2=-35.1 kJ·mol-1
③HCl(g)+H2O(l)===HCl(aq) ΔH3=-72.3 kJ·mol-1
④NH4Cl(s)+H2O(l)===NH4Cl(aq) ΔH4=?
⑤NH3·H2O(aq)+HCl(aq)===NH4Cl(aq)+H2O(l) ΔH5=-52.3 kJ·mol-1
(1)如图所示,组成一个原电池.

①当电解质溶液为稀硫酸时:
Cu电极是_____(填“正”或“负”)极,其电极反应为____;
②当电解质溶液为浓硝酸时:
Cu电极是_____极,其电极反应为__________。
(2)请写出电解硫酸铜溶液的总化学方程式 。
(3)燃烧氢气时耗氧量小,放出热量多。已知4g H2燃烧生成液态水时放热为571.6kJ,试写出表示H2燃烧热的热化学方程式为: 。
(4)下图是一碳酸盐燃料电池(MCFC),以水煤气(CO、H2)为燃料,一定比例Li2CO3和Na2CO3低熔混合物为电解质。写出B极发生的电极反应式: 。

(5)请根据下面所给出的5个热化学方程式,判断反应④的反应热ΔH4是________。
①NH3(g)+HCl(g)===NH4Cl(s) ΔH1=-176 kJ·mol-1
②NH3(g)+H2O(l)===NH3·H2O(aq) ΔH2=-35.1 kJ·mol-1
③HCl(g)+H2O(l)===HCl(aq) ΔH3=-72.3 kJ·mol-1
④NH4Cl(s)+H2O(l)===NH4Cl(aq) ΔH4=?
⑤NH3·H2O(aq)+HCl(aq)===NH4Cl(aq)+H2O(l) ΔH5=-52.3 kJ·mol-1