网址:http://m.1010jiajiao.com/timu3_id_81424[举报]
(1)在25℃、101kPa下,16g甲烷燃烧生成CO2和液态水时放热889.6kJ。则表示甲烷燃烧的热化学方程式为_________________________________________________。
(2) 下列说法正确的是(填序号)__ __:(漏选得1分,错选、多选得0分)
| A.相同温度下,0.1 mol?L-1NH4Cl溶液中NH4+的浓度比0.1 mol?L-1氨水中NH4+的浓度大; |
| B.用稀盐酸洗涤AgCl沉淀比用水洗涤损耗AgCl小; |
| C.电解饱和食盐水时,阳极得到氢氧化钠溶液和氢气; |
D.对于Al(OH)3(s) Al(OH)3(aq) Al(OH)3(aq) Al3+(aq)+3OH-(aq),前段为溶解平衡,后段是电 Al3+(aq)+3OH-(aq),前段为溶解平衡,后段是电 |
E. 除去溶液中的Mg2+,用OH-沉淀Mg2+比用CO32-效果好,说明Mg(OH)2的溶解度比
MgCO3的大; 查看习题详情和答案>>
(1)室温时氢氧化钙的溶度积KSP =4.7×10-6, 室温时将9 mL0.02 mol·L—1的氯化钙溶液与1 mL
pH=13的氢氧化钠溶液混合后(溶液体积可直接加和),溶液中 沉淀析出(填有或无).
(2)2.24升(标准状态)氨气通入250mL 浓度为0.1 mol·L—1的硫酸溶液中,充分反应后溶液中各离子浓度大小为 (按由大到小的顺序写出各离子符号)
(3) 人体血液含Ca2+。现抽取某人血样10 mL,稀释后用草酸铵[(NH4)2C2O4]溶液处理,使Ca2+完全转变为草酸钙(CaC2O4)沉淀;过滤,洗涤,将得到的沉淀用稀硫酸溶解得到草酸(H2C2O4)溶液,用5.0×10-3 mol·L-1 KMnO4溶液滴定得到的草酸至终点,共耗去2.0 mL KMnO4溶液。由上述实验数据可求得此人血液中Ca2+的物质的量浓度为 .(提示:2 KMnO4 + 5H2C2O4 + 3H2SO4 = 2MnSO4 + K2SO4 + 10CO2↑+8H2O)
(4)用下图完成相应实验.

①断开K1,闭合K2接通直流电源,写出阳极 的电极反应 .
电解的总反应方程式
②电解一段时间后,当两石墨棒均有气体包围时,切断K2闭合K1,发现电路中有电流通过. 写出a极的电极反应式
查看习题详情和答案>>(12分)惰性电极电解NaCl溶液或CuSO4溶液都得到三种产物A、B、C,各物质之间的转化关系如下图所示(图中参与反应和生成的水都已略去)。已知甲是短周期元素的单质,它是日常生活中常用的包装材料。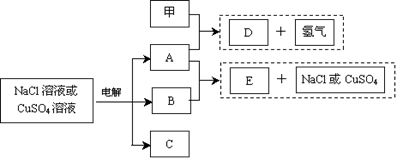
回答下列问题:
(1)若电解的是NaCl溶液:
①甲与A反应的化学方程式是 。
②A与B反应的离子方程式是 。
③常温下,若电解100mL 0.1 mol/L NaCl溶液,阴、阳两极各产生112mL气体(标准状况),则所得溶液的pH为 (忽略反应前后溶液的体积变化及气体溶于水的影响)。
(2)若 电解的是CuSO4溶液,加热时,A的浓溶液可与B发生反应:
电解的是CuSO4溶液,加热时,A的浓溶液可与B发生反应:
①A的浓溶液与B反应过程中,A的浓度随时间变化的图像正确是 。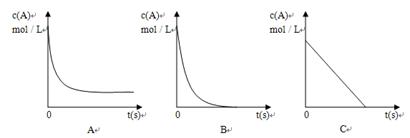
②E的化学式是 ;电解时阳极的电极反应式是 。
(1)在25℃、101kPa下,16g甲烷燃烧生成CO2和液态水时放热889.6kJ。则表示甲烷燃烧的热化学方程式为_________________________________________________。
(2) 下列说法正确的是(填序号)__ __:(漏选得1分,错选、多选得0分)
A.相同温度下,0.1 mol?L-1NH4Cl溶液中NH4+的浓度比0.1 mol?L-1氨水中NH4+的浓度大;
B.用稀盐酸洗涤AgCl沉淀比用水洗涤损耗AgCl小;
C.电解饱和食盐水时,阳极得到氢氧化钠溶液和氢气;
D.对于Al(OH)3(s) Al(OH)3(aq)
Al(OH)3(aq) Al3+(aq)+3OH-(aq),前段为溶解平衡,后段是电
Al3+(aq)+3OH-(aq),前段为溶解平衡,后段是电
离平衡;
E. 除去溶液中的Mg2+,用OH-沉淀Mg2+比用CO32-效果好,说明Mg(OH)2的溶解度比
MgCO3的大;
查看习题详情和答案>>