摘要:0.05 +O2(g)===SO2(g) ΔH1=-296 kJ·mol-1 SO2(g)+O2(g)===SO3(g) ΔH2=-99 kJ·mol-1 3S(s)+O2(g)===3SO3(g) ΔH=(ΔH1+ΔH2)×3=-1 185 kJ·mol-1
网址:http://m.1010jiajiao.com/timu3_id_80862[举报]
已知:体系的自由能变化ΔG=ΔH-TΔS<0时,反应能自发进行。下列反应中,一定不能自发进行的是( )
| A.2KClO3(s)=2KCl(s)+3O2(g) ΔH=-78.03kJ/mol ΔS="1110" J/(mol·K) |
| B.CO(g)=C(s,石墨)+1/2O2(g) ΔH=110.5kJ/mol ΔS="-89.36" J/(mol·K) |
| C.4Fe(OH)2(s)+2H2O(l)+O2(g)=4Fe(OH)3(s) ΔH=-444.3kJ/molΔS="-280.1" J/(mol·K) |
| D.NH4HCO3(s)+CH3COOH(aq)=CO2(g)+CH3COONH4(aq)+H2O(l) |
已知:体系的自由能变化ΔG=ΔH-TΔS<0时,反应能自发进行。下列反应中,一定不能自发进行的是( )
A.2KClO3(s)=2KCl(s)+3O2(g) ΔH=-78.03kJ/mol ΔS="1110" J/(mol·K)
B.CO(g)=C(s,石墨)+1/2O2(g) ΔH=110.5kJ/mol ΔS="-89.36" J/(mol·K)
C.4Fe(OH)2(s)+2H2O(l)+O2(g)=4Fe(OH)3(s) ΔH=-444.3kJ/molΔS="-280.1" J/(mol·K)
D.NH4HCO3(s)+CH3COOH(aq)=CO2(g)+CH3COONH4(aq)+H2O(l)
ΔH=37.301kJ/mol ΔS="184.05" J/(mol·K)
查看习题详情和答案>>
下列解释原理的表达式中,不正确的是( )
A、镁的冶炼:MgCl2(熔融)
| ||||
B、用热的纯碱溶液清洗油污:CO
| ||||
C、用Na2CO3溶液处理水垢中的CaSO4:CaSO4(s)+CO
| ||||
| D、用氢氟酸刻蚀玻璃:4HF+SiO2=SiF4↑+2H2O |
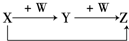 X、Y、Z、W有如图所示的转化关系,则X、W可能是①C、O2 ②AlCl3、NaOH ③Fe、HNO3 ④S、O2( )
X、Y、Z、W有如图所示的转化关系,则X、W可能是①C、O2 ②AlCl3、NaOH ③Fe、HNO3 ④S、O2( )