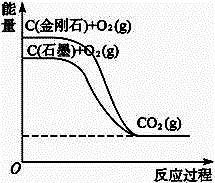
摘要:5.分析下图所示各物质能量变化关系.下列热化学方程式正确的是 ( ) A.C+O2(g)===CO2(g),ΔH1=a kJ·mol-1 B.C+O2(g)===CO2(g),ΔH2=b kJ·mol-1(b>0) C.C+O2===CO2(g),ΔH=c kJ·mol-1(c>0) D.C,ΔH4=d kJ·mol-1(d>0) [答案] A
网址:http://m.1010jiajiao.com/timu3_id_72728[举报]
分析下图所示各物质能量变化关系,下列热化学方程式正确的是( )?

A.C(金刚石)+O2(g) ![]() CO2(g) ΔH1=a kJ·mol-1?(a<0)?
CO2(g) ΔH1=a kJ·mol-1?(a<0)?
B.C(石墨)+O2(g) ![]() CO2(g) ΔH=b kJ·mol-1?(b>0)?
CO2(g) ΔH=b kJ·mol-1?(b>0)?
C.C+O2![]() CO2(g) ΔH3=c kJ·mol-1?(c<0)?
CO2(g) ΔH3=c kJ·mol-1?(c<0)?
D.C(金刚石) ![]() C(石墨) ΔH4=dkJ·mol-1 (d>0)?
C(石墨) ΔH4=dkJ·mol-1 (d>0)?
分析下图所示各物质能量变化关系,下列热化学方程式正确的是( )

A.C (金刚石)+O2(g)====CO2(g);ΔH1=a kJ·mol-1 (a<0)
B.C (石墨)+O2(g)====CO2(g);ΔH2=b kJ·mol-1 (b>0)
C.C +O2====CO2(g);ΔH3=c kJ·mol-1 (c<0)
D.C (金刚石)====C (石墨);ΔH4=d kJ·mol-1 (d>0)
查看习题详情和答案>>
分析下图所示各物质能量变化关系,下列热化学方程式正确的是?

A.C(金刚石)+O2(g)=CO2(g);ΔH1 =a kJ·mol-1(a<0)??
B.C(石墨)+O2(g)=CO2(g);ΔH2 =b kJ·mol-1(b>0)?
C.C+O2=CO2(g);ΔH3=c kJ·mol-1(c<0)?
D.C(金刚石)= C(石墨);ΔH4=d kJ·mol-1(d>0)
查看习题详情和答案>>
 CO2(g) ΔH1=a kJ·mol-1?(a<0)?
CO2(g) ΔH1=a kJ·mol-1?(a<0)?