网址:http://m.1010jiajiao.com/timu3_id_63258[举报]
已知25℃时,AgI饱和溶液中c(Ag+)为1.23×10-8mol/L,AgCl饱和溶液中c(Ag+)为1.25×10-5mol/L,若在5mL含有KCl、KI各为0.01mol/L的溶液中加入8mL0.01mol/LAgNO3溶液,则下列叙述正确的
A.混合溶液中c(K+)>c(NO3-)>c(Ag+)>c(Cl-)>c(I-)
B.混合溶液中c(K+)>c(NO3-)>c(Cl-)>c(Ag+)>c(I-)
C.加入AgNO3溶液时首先生成AgCl沉淀
D.混合溶液中约为1.02×10-3
查看习题详情和答案>>
已知25℃时,AgI饱和溶液中c(Ag+)为1.22×10-8mol/L,AgCl的饱和溶液中c(Ag+)为1.25×10-5mol/L。若在5mL含有KCl和KI各为0.01mol/L的溶液中,加入8mL0.01mol/LAgNO3
溶液,这时溶液中所含溶质的离子浓度大小关系正确的是( )
A.c (K+)>c (NO3-) >c (Ag+) = c (Cl-) + c (I-)
B.c (K+)>c (NO3-) >c (Ag+) >c (Cl-)>c (I-)
C.c (NO3-)>c (K+)>c (Ag+) >c (Cl-)>c (I-)
D.c (K+)>c (NO3-) >c (Cl-) >c (Ag+)>c (I-)
查看习题详情和答案>>
已知25℃时,AgI饱和溶液中c(Ag+)为1.23×10-8mol/L,AgCl饱和溶液中c(Ag+)为1.25×10-5mol/L,若在5mL含有KCl、KI各为0.01mol/L的溶液中加入8mL0.01mol/LAgNO3溶液,则下列叙述正确的
A.混合溶液中c(K+)>c(NO3-)>c(Ag+)>c(Cl-)>c(I-)
B.混合溶液中c(K+)>c(NO3-)>c(Cl-)>c(Ag+)>c(I-)
C.加入AgNO3溶液时首先生成AgCl沉淀
D.混合溶液中 约为1.02×10-3
约为1.02×10-3
查看习题详情和答案>>
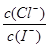 约为1.02×103
约为1.02×103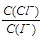 约为1.02×10-3
约为1.02×10-3