摘要:化学反应N2+3H2 = 2NH3的能量变化如题13图所示.该反应的热化学方程式是 A.N2(g)+3H2(g) = 2NH3kJ/mol B.N2(g)+3H2(g) = 2NH3kJ/mol C.N2(g)+H2(g) = NH3kJ/mol D.N2(g)+H2(g) =NH3kJ/mol
网址:http://m.1010jiajiao.com/timu3_id_47088[举报]
化学反应N2+3H2=2NH3的能量变化如图所示,E是正值,该反应的热化学方程式是( )
| A.N2(g)+3H2(g)=2NH3(1); △H=2(a-b-c)kJ·mol-1 |
| B.N2(g)+3H2(g)=2NH3(g);△H=2(b-a)kJ·mol-1 |
C. N2(g)+ N2(g)+ H2(g)=NH3(1);△H=(b+c-a)kJ·mol-1 H2(g)=NH3(1);△H=(b+c-a)kJ·mol-1 |
D. N2(g)+ N2(g)+ H2(g)=NH3(g); △H=(a+b)kJ·mol H2(g)=NH3(g); △H=(a+b)kJ·mol |
化学反应N2+3H2=2NH3的能量变化如图所示,E是正值,该反应的热化学方程式是( )

A.N2(g)+3H2(g)=2NH3(1); △H=2(a-b-c)kJ·mol-1
B.N2(g)+3H2(g)=2NH3(g);△H=2(b-a)kJ·mol-1
C. N2(g)+
N2(g)+ H2(g)=NH3(1);△H=(b+c-a)kJ·mol-1
H2(g)=NH3(1);△H=(b+c-a)kJ·mol-1
D. N2(g)+
N2(g)+ H2(g)=NH3(g);
△H=(a+b)kJ·mol
H2(g)=NH3(g);
△H=(a+b)kJ·mol
查看习题详情和答案>>
化学反应N2+3H2=2NH3的能量变化如图所示,E是正值,该反应的热化学方程式是( )

A.N2(g)+3H2(g)=2NH3(1); △H=2(a-b-c)kJ·mol-1
B. N2(g)+3H2(g)=2NH3(g);△H=2(b-a)kJ·mol-1
C.![]() N2(g)+
N2(g)+![]() H2(g)=NH3(1);△H=(b+c-a)kJ·mol-1
H2(g)=NH3(1);△H=(b+c-a)kJ·mol-1
D.![]() N2(g)+
N2(g)+![]() H2(g)=NH3(g); △H=(a+b)kJ·mol
H2(g)=NH3(g); △H=(a+b)kJ·mol
查看习题详情和答案>>


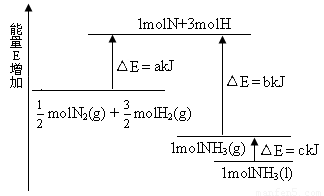
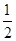 N2(g)+
N2(g)+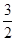 H2(g) === NH3(1) △H=(b+c-a)kJ·mol-1
H2(g) === NH3(1) △H=(b+c-a)kJ·mol-1