摘要:16.25℃.101kPa下.碳.氢气和甲烷燃烧热依次是393.5kJ/mol.285.8kJ/mol.890.3kJ/mol.则下列热化学方程式正确的是 ( ) A.2H2(g)+O2(g)=2H2O(l),△H=-571.6kJ/mol B.2H2(g)+O2(g)=2H2O(g),△H=-571.6kJ/mol C.CH4(g)+2O2(g)=CO2(g)+2H2O(g),△H=-890.3kJ/mol D.C(s)+1/2O2,△H=-393.5kJ/mol
网址:http://m.1010jiajiao.com/timu3_id_425674[举报]
25℃、101kPa下,碳、氢气和甲烷的燃烧热依次是393.5 kJ·mol—1、285.8 kJ·mol—1
和890.3kJ·mol—1,下列选项中正确的是
| A.2H2(g)+O2(g)=2H2O(l)△H=—285.8kJ·mol—1 |
| B.CH4(g)+2O2(g)=CO2(g)+2H2O(l)△H=+890.3kJ·mol—1 |
| C.H2(g)+1/2O2(g)=H2O(g)△H<—285.8kJ·mol—1 |
| D.C(s)+2H2(g)=CH4(g)△H=—74.8 kJ·mol—1 |
25℃、101kPa下,碳、氢气和甲烷的燃烧热依次是393.5 kJ·mol—1、285.8 kJ·mol—1
和890.3kJ·mol—1,下列选项中正确的是
和890.3kJ·mol—1,下列选项中正确的是
| A.2H2(g)+O2(g)=2H2O(l)△H=—285.8kJ·mol—1 |
| B.CH4(g)+2O2(g)=CO2(g)+2H2O(l)△H=+890.3kJ·mol—1 |
| C.H2(g)+1/2O2(g)=H2O(g)△H<—285.8kJ·mol—1 |
| D.C(s)+2H2(g)=CH4(g)△H=—74.8 kJ·mol—1 |
25℃、101kPa下,碳、氢气、甲烷和葡萄糖的燃烧热依次是393.5kJ/mol、285.8kJ/mol、890.3kJ/mol、2800kJ/mol,则下列热化学方程式正确的是( )
25℃、101kPa下,碳、氢气、甲烷和葡萄糖的燃烧热依次是ΔH=-393.5kJ/mol、ΔH=-285.8kJ/mol、ΔH=-890.3kJ/mol、ΔH=-2800kJ/mol,则下列热化学方程式正确的是( )
A.C(s)+ O2(g)===CO(g)ΔH=-393.5kJ/mol O2(g)===CO(g)ΔH=-393.5kJ/mol |
| B.2H2(g)+O2(g)===2H2O(g)ΔH=+571.6kJ/mol |
| C.CH4(g)+2O2(g)===CO2(g)+2H2O(g)ΔH=-890.3kJ/mol |
D. C6H12O6(s)+3O2(g)===3CO2(g)+3H2O(l)ΔH=-1400kJ/mol C6H12O6(s)+3O2(g)===3CO2(g)+3H2O(l)ΔH=-1400kJ/mol |
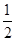 O2(g)===CO(g)ΔH=-393.5kJ/mol
O2(g)===CO(g)ΔH=-393.5kJ/mol