摘要:图7.图8.图9.图10.图11.图12.图13中两灯并联.S是总开关.S1只控制灯泡L1.请将所缺的导线补上.
网址:http://m.1010jiajiao.com/timu3_id_3977197[举报]
11、元素周期表是我们学习和研究化学的重要工具,它的内容十分丰富.下面是元素周期表部分内容,请回答下列问题:
(1)由图中可得出,原子序数(提示:原子序数=核内质子数)为8的元素的原子结构示意图为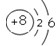 ,该元素的原子在化学反应中易
,该元素的原子在化学反应中易
(2)写出原子序数为1、6、8、11四种元素所组成的一种化合物的化学式
查看习题详情和答案>>
| 1 | 1 H 氢 1.008 |
2 He 氦 4.003 | ||||||
| 2 | 3 Li 锂 6.941 |
4 Be 铍 9.012 |
5 B 硼 10.81 |
6 C 碳 12.01 |
7 N 氮 14.01 |
8 O 氧 16.00 |
9 F 氟 19.00 |
10 Ne 氖 20.18 |
| 3 | 11 Na 钠 22.99 |
12 Mg 镁 24.31 |
13 Al 铝 26.98 |
14 Si 硅 28.09 |
15 P 磷 30.97 |
16 S 硫 32.06 |
17 Cl 氯 35.45 |
18 Ar 氩 39.95 |
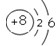
得到
(选填“得到”或者“失去”)电子.(2)写出原子序数为1、6、8、11四种元素所组成的一种化合物的化学式
NaHCO3
.纯碱长期暴露在空气中能吸收空气中的水分及二氧化碳,生成碳酸氢钠,并结成硬块.写出该反应的化学方程式:Na2CO3+H2O+CO2=2NaHCO3
.21、图A是元素周期表的一部分

(1)原子序数为4的元素名称为
(2)图B是某元素的原子结构示意图,该元素在图A中的位置是(填①或②或③)
(4)图C是氯元素的原子结构示意图,在图A中找出比氯元素少8个质子的元素,并试画出该元素的离子结构示意图 .
.
(5)图A中②号元素与第17号元素组成的化合物的化学式为
查看习题详情和答案>>
| 族/周期 | ⅠA | ⅡA | ⅢA | ⅣA | ⅤA | ⅥA | ⅦA | 0 |
| 第二 周期 |
3 Li 锂 6.941 |
4 Be 铍 9.012 |
5 B 硼 10.8l |
① | 7 N 氮 14.0l |
8 O 氧 16.00 |
9 F 氟 19.00 |
10 Ne 氖 20.18 |
| 第三 周期 |
11 Na 钠 22.99 |
② 24.31 |
13 Al 铝 26.98 |
14 Si 硅 28.09 |
③ 30.97 |
16 S 硫 32.06 |
17 Cl 氯 35.45 |
18 Ar 氩 39.95 |

(1)原子序数为4的元素名称为
铍
;(2)图B是某元素的原子结构示意图,该元素在图A中的位置是(填①或②或③)
②
;(3)相对原子质量为30.97的元素属非金属
(填“金属”或“非金属”),你认为位置①中的相对原子质量约为12
(填整数值).(4)图C是氯元素的原子结构示意图,在图A中找出比氯元素少8个质子的元素,并试画出该元素的离子结构示意图

(5)图A中②号元素与第17号元素组成的化合物的化学式为
MgCl2
.26、图1是元素周期表的一部分
试根据上表回答下列问题:
(1)氮元素的核电荷数x=
(2)图2是某元素的原子结构示意图,该元素在图1中的位置是(填①或②或③)
(3)图3是氯元素的原子结构示意图,图1中
(4)第二周期元素中分子中含有两个原子物质的化学式
(5)分析上表可发现:每一横行元素从左向右排列所遵循的一条规律是
查看习题详情和答案>>
| 族 周期 |
ⅠA | ⅡA | ⅢA | ⅣA | ⅤA | ⅥA | ⅦA | 0 |
| 第二 周期 |
3 Li 锂 7 |
4 Be 铍 9 |
5 B 硼 11 |
① | 7 N 氮 14 |
8 O 氧 16 |
9 F 氟 19 |
10 Ne 氖 20 |
| 第三 周期 |
11 Na 钠 23 |
② | 13 Al 铝 27 |
14 Si 硅 28 |
③ | 16 S 硫 32 |
17 Cl 氯 35.5 |
18 Ar 氩 40 |

(1)氮元素的核电荷数x=
7
.(2)图2是某元素的原子结构示意图,该元素在图1中的位置是(填①或②或③)
①
;(3)图3是氯元素的原子结构示意图,图1中
F
元素与氯元素化学性质相似.(4)第二周期元素中分子中含有两个原子物质的化学式
O2
;(5)分析上表可发现:每一横行元素从左向右排列所遵循的一条规律是
每一横行质子数逐渐增大
.(9分)我国制碱工业的先驱侯德榜将制碱与制氨结合起来的联合制碱法,为纯碱和氮肥工业技术的发展做出了杰出的贡献。其生产工艺流程示意图如下:
【资料】四种盐在不同温度下的溶解度表
| | 10℃ | 20℃ | 30℃ | 40℃ | 50℃ |
| NaCl | 35.8 | 36.0 | 36.3 | 36.6 | 37.0 |
| NH4HCO3 | 15.8 | 21.0 | 27.0 | —— | —— |
| NaHCO3 | 8.1 | 9.6 | 11.1 | 12.7 | —— |
| NH4Cl | 33.3 | 37.2 | 41.4 | 45.8 | 50.4 |
⑵操作a是 ,生产流程中被循环利用的物质是 ;
⑶在精盐水中先通入NH3,使溶液呈 性,再通入CO2,有利于对CO2的吸收;
⑷氨盐水中通入二氧化碳后,在常温条件下,易析出NaHCO3晶体而不析出NH4Cl晶体的可能原因是 ;
⑸粗盐水中主要含有CaCl2、MgCl2等杂质,工业上常加入下列物质除杂、精制,则加入下列三种试剂合理的顺序为 (填序号);
A.适量的盐酸 B.稍过量的Na2CO3溶液 C.稍过量的Ca(OH)2溶液
⑹副产品NH4Cl可做 肥,若加入氢氧化钠加热,反应的化学方程式为 。 查看习题详情和答案>>