摘要:13.已知H-H键能为436 kJ·mol-1.H-N键能为391 kJ·mol-1.根据化学方程式:N2 + 3H2 = 2NH3 ΔH = -92.4 kJ·mol-1.则N≡N键的键能是( ) A.431 kJ·mol-1 B.946 kJ·mol-1 C.649 kJ·mol-1 D.869 kJ·mol-1
网址:http://m.1010jiajiao.com/timu3_id_314194[举报]
已知H—H键能为436 kJ·mol-1,H—N键能为391 kJ·mol-1,根据化学方程式N2+3H2 2NH3 ΔH=-92.4 kJ·mol-1,则N≡N的键能是( )
2NH3 ΔH=-92.4 kJ·mol-1,则N≡N的键能是( )
A.431 kJ·mol-1 B.945.6 kJ·mol
已知H—H键能为436 kJ·mol-1,H—N键能为391 kJ·mol-1,根据化学方程式:N2+3H2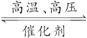 2NH3 ΔH=-92.4 kJ·mol-1,则N≡N键的键能是( )
2NH3 ΔH=-92.4 kJ·mol-1,则N≡N键的键能是( )
A.431 kJ·mol-1 B.946 kJ·mol
已知H—H键能为436 kJ·mol-1,H—N键能为391 kJ·mol-1,根据化学方程式:N2+3H2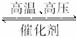 2NH3,1 mol N2反应放出的热量为92.4 kJ。则N≡N键的键能是( )
2NH3,1 mol N2反应放出的热量为92.4 kJ。则N≡N键的键能是( )
A.431 kJ·mol-1 B.945 kJ·mol