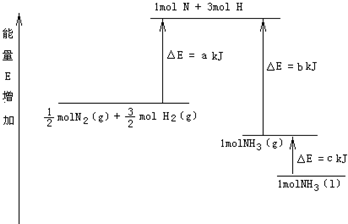
摘要:8.化学反应N2+3H2=2NH3的能量变化如图所示.E是正值.该反应的热化学方程式是( ) A.N2(g)+3H2(g)=2NH3kJ·mol-1 B. N2(g)+3H2(g)=2NH3kJ·mol-1 C.N2(g)+H2(g)=NH3kJ·mol-1 D.N2(g)+H2(g)=NH3kJ·mol-1
网址:http://m.1010jiajiao.com/timu3_id_304171[举报]
化学反应N2+3H2?2NH3的能量变化如图所示,该反应的热化学方程式是( )
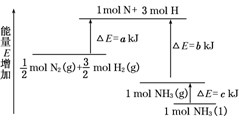

| A、N2(g)+3H2(g)?2NH3(l);△H=2(a-b-c) kJ/mol | ||||
| B、N2(g)+3H2(g)?2NH3(g);△H=2(b-a) kJ/mol | ||||
C、
| ||||
D、
|
 化学反应N2+3H2→2NH3的能量变化如图所示,该反应的热化学方程式是( )
化学反应N2+3H2→2NH3的能量变化如图所示,该反应的热化学方程式是( )| A、N2(g)+H2(g)→NH3(1)-46 kJ | B、N2(g)+H2(g)→NH3(g)-454 kJ | C、N2(g)+3 H2(g)→2 NH3(g)+92 kJ | D、N2(g)+3 H2(g)→2 NH3(1)+431.3 kJ |