摘要:68.已知在1×105 Pa.298 K条件下.2mol氢气燃烧生成水蒸气放出484 kJ热量.下列热化学方程式正确的是( ) A.H2O(g) = H2(g) + O2 (g) △H = +242 kJ/mol B.2H2(g) + O2 (g) = 2H2O (l) △H = -484 kJ/mol C.H2 (g) + O2 (g) = H2O (l) △H = -242 kJ/mol D.2H2 (g) + O2 (g) = 2H2O (g) △H = +484 kJ/mol
网址:http://m.1010jiajiao.com/timu3_id_294967[举报]
已知在1×105 Pa,298 K条件下,2mol氢气燃烧生成水蒸气放出484 kJ热量,下列热化学方程式正确的是( )
A.H2O(g) = H2(g) + ![]() O2 (g) △H = +242 kJ/mol
O2 (g) △H = +242 kJ/mol
B.2H2(g) + O2 (g) = 2H2O (l) △H = -484 kJ/mol
C.H2 (g) + ![]() O2 (g) = H2O (l) △H = -242 kJ/mol
O2 (g) = H2O (l) △H = -242 kJ/mol
D.2H2 (g) + O2 (g) = 2H2O (g) △H = +484 kJ/mol
查看习题详情和答案>>已知在1×105 Pa,298 K条件下,2mol氢气燃烧生成水蒸气放出484 kJ热量,下
列热化学方程式正确的是( )
A.H2O(g) = H2(g) + ![]() O2 (g) △H = +242 kJ/mol
O2 (g) △H = +242 kJ/mol
B.2H2(g) + O2 (g) = 2H2O (l) △H = -484 kJ/mol
C.H2 (g) + ![]() O2 (g) = H2O (l) △H = -242 kJ/mol
O2 (g) = H2O (l) △H = -242 kJ/mol
D.2H2 (g) + O2 (g) = 2H2O (g) △H = +484 kJ/mol
查看习题详情和答案>>已知在1×105 Pa,298 K条件下,2mol氢气燃烧生成水蒸气放出484 kJ热量,下
列热化学方程式正确的是
A.H2O(g)= H2(g)+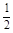 O2 (g)△H =+242 kJ/mol O2 (g)△H =+242 kJ/mol |
| B.2H2(g)+ O2 (g)= 2H2O (l)△H =-484 kJ/mol[来 |
C.H2 (g)+ 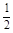 O2 (g) O2 (g) = H2O = H2O (l)△H =-242 kJ/mol (l)△H =-242 kJ/mol |
| D.2H2 (g)+ O2 (g)= 2H2O (g)△H =+484 kJ/mol |