网址:http://m.1010jiajiao.com/timu3_id_274206[举报]
已知下列热化学方程式:
 CH4(g)+O2(g)=
CH4(g)+O2(g)=
 C02(g)+H2O(1);∆H
=-445.15kJ·mol-1
C02(g)+H2O(1);∆H
=-445.15kJ·mol-1
CH4(g)+ O2(g)=CO(g)+2H2O(1);
∆H=-607.3kJ·mol-1
O2(g)=CO(g)+2H2O(1);
∆H=-607.3kJ·mol-1
CH4(g)+2O2(g)=CO2(g)+2H2O(1); ∆H =-890.3 kJ·mol-1
CH4(g)+2O2(g)=CO2(g)十2H2O(g); ∆H =-802.3 kJ·mol-1
则CH4的燃烧热为
A.445.15 kJ·mol-1 B.607.3 kJ·mol-1 C.890.3 kJ·mol-1 D.802.3 kJ·mol-1
查看习题详情和答案>>
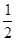 CH4(g)+O2(g)=
CH4(g)+O2(g)= 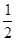 C02(g)+H2O(1);?H =-445.15kJ·mol-1
C02(g)+H2O(1);?H =-445.15kJ·mol-1CH4(g)+
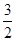 O2(g)=CO(g)+2H2O(1); ?H=-607.3kJ·mol-1
O2(g)=CO(g)+2H2O(1); ?H=-607.3kJ·mol-1CH4(g)+2O2(g)=CO2(g)+2H2O(1); ?H ="-890.3" kJ·mol-1
CH4(g)+2O2(g)=CO2(g)十2H2O(g); ?H ="-802.3" kJ·mol-1
则CH4的燃烧热为
| A.445.15 kJ·mol-1 | B.607.3 kJ·mol-1 | C.890.3 kJ·mol-1 | D.802.3 kJ·mol-1 |
已知下列热化学方程式: CH4(g)+O2(g)=
CH4(g)+O2(g)=  C02(g)+H2O(1);?H =-445.15kJ·mol-1
C02(g)+H2O(1);?H =-445.15kJ·mol-1
CH4(g)+ O2(g)=CO(g)+2H2O(1); ?H=-607.3kJ·mol-1
O2(g)=CO(g)+2H2O(1); ?H=-607.3kJ·mol-1
CH4(g)+2O2(g)=CO2(g)+2H2O(1); ?H ="-890.3" kJ·mol-1
CH4(g)+2O2(g)=CO2(g)十2H2O(g); ?H ="-802.3" kJ·mol-1
则CH4的燃烧热为
| A.445.15 kJ·mol-1 | B.607.3 kJ·mol-1 | C.890.3 kJ·mol-1 | D.802.3 kJ·mol-1 |
能源是国民经济发展的重要基础,我国目前使用的能源主要是化石燃料。
(1)在25 ℃、101 kPa时,8 g CH4完全燃烧生成液态水时放出的热量是445.15 kJ,则CH4燃烧的热化学方程式是 。
(2)已知:C(s) + O2(g)  CO2(g) ΔH=-437.3 kJ?mol-1
CO2(g) ΔH=-437.3 kJ?mol-1
H2(g) + 1/2 O2(g)  H2O(g) ΔH=-285.8 kJ?mol-1
H2O(g) ΔH=-285.8 kJ?mol-1
CO(g) + 1/2 O2(g)  CO2(g) ΔH=-283.0 kJ?mol-1
CO2(g) ΔH=-283.0 kJ?mol-1
则煤的气化主要反应的热化学方程式是:C(s) + H2O(g)  CO(g) + H2(g) ΔH= kJ?mol-1。如果该反应ΔS=+133.7 J·K-1·mol-1 该反应在常温(25 ℃)下能否自发进行?(△G=△H-T△S) (填“能”或“不能”,并写出判断依据).
CO(g) + H2(g) ΔH= kJ?mol-1。如果该反应ΔS=+133.7 J·K-1·mol-1 该反应在常温(25 ℃)下能否自发进行?(△G=△H-T△S) (填“能”或“不能”,并写出判断依据).
(3)由气态基态原子形成1mol化学键释放的最低能量叫键能。从化学键的角度分析,化学反应的过程就是反应物的化学键的破坏和生成物的化学键的形成过程。在化学反应过程中,拆开化学键需要消耗能量,形成化学键又会释放能量。
| 化学键 | H-H | N-H | N≡N |
| 键能/kJ·mol-1 | 436 | 391 | 945 |
 2NH3 △H="a" kJ·mol-1。试根据表中所列键能数据估算a的数值为 。
查看习题详情和答案>>
2NH3 △H="a" kJ·mol-1。试根据表中所列键能数据估算a的数值为 。
查看习题详情和答案>>
(1)在25 ℃、101 kPa时,8 g CH4完全燃烧生成液态水时放出的热量是445.15 kJ,则CH4燃烧的热化学方程式是 。
(2)已知:C(s) + O2(g)
 CO2(g) ΔH=-437.3 kJ?mol-1
CO2(g) ΔH=-437.3 kJ?mol-1H2(g) + 1/2 O2(g)
 H2O(g) ΔH=-285.8 kJ?mol-1
H2O(g) ΔH=-285.8 kJ?mol-1CO(g) + 1/2 O2(g)
 CO2(g) ΔH=-283.0 kJ?mol-1
CO2(g) ΔH=-283.0 kJ?mol-1则煤的气化主要反应的热化学方程式是:C(s) + H2O(g)
 CO(g) + H2(g) ΔH= kJ?mol-1。如果该反应ΔS=+133.7 J·K-1·mol-1 该反应在常温(25 ℃)下能否自发进行?(△G=△H-T△S) (填“能”或“不能”,并写出判断依据).
CO(g) + H2(g) ΔH= kJ?mol-1。如果该反应ΔS=+133.7 J·K-1·mol-1 该反应在常温(25 ℃)下能否自发进行?(△G=△H-T△S) (填“能”或“不能”,并写出判断依据).(3)由气态基态原子形成1mol化学键释放的最低能量叫键能。从化学键的角度分析,化学反应的过程就是反应物的化学键的破坏和生成物的化学键的形成过程。在化学反应过程中,拆开化学键需要消耗能量,形成化学键又会释放能量。
| 化学键 | H-H | N-H | N≡N |
| 键能/kJ·mol-1 | 436 | 391 | 945 |
 2NH3 △H="a" kJ·mol-1。试根据表中所列键能数据估算a的数值为 。
2NH3 △H="a" kJ·mol-1。试根据表中所列键能数据估算a的数值为 。