网址:http://m.1010jiajiao.com/timu3_id_273182[举报]
| A.如果设“此”为电解质,“彼”为非电解质,不是所有的物质都是非此即彼的 |
| B.硅便于运输、贮存,燃烧放出的热量大,将是“21世纪的最佳能源” |
| C.相同温度下,同一化学反应的化学计量系数不同,平衡常数的值可能也不同 |
| D.在迄今发现的元素中,不一定所有非金属元素(稀有气体除外)都满足条件“主族元素的族序数≥其周期序数” |
(13分)

一种用途较广泛的有机玻璃树脂聚丁烯酸甲酯,合成这种高聚物有多种途径,有一种合成途径的副产品污染少或无污染,原子利用率较高,符合“绿色化学”的要求,其合成路线如下:

试解答下列问题:
(1)写出A代表的有机物结构简式: (2分)
(2)写出E的两种羧酸类同分异构体的结构简式: 、 (4分)
(3)在上述反应中,原子利用率最高的化学反应(填写序号): (2分)
(4)此合成途径:②的化学反应类型: (1分) ④的化学反应类型: (1分)
(5)写出⑤的化学方程式 (2分)
(6)聚丁烯酸甲酯__________固定的沸点(填“有”或“没有”)。(1分)
查看习题详情和答案>>(10分)在一容积为2 L的密闭容器中,加入0.2 mol的N2和0.6 mol的H2,在一定条件下发生反应:N2(g)+3H2(g)  2NH3(g) ΔH<0
2NH3(g) ΔH<0
反应中NH3的物质的量浓度的变化情况如下图所示,请回答下列问题: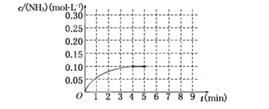
(1)根据上图,计算从反应开始到平衡时,平均反应速率v(NH3)为______________.
(2)该反应达到平衡时H2的转化率________.
(3)反应达到平衡后,第5分钟末,保持其它条件不变,若改变反应温度,达到新平衡时NH3的物质的量浓度不可能为____________.(填序号)
A、0.20 mol·L-1 b、0.12 mol·L-1 c、0.10 mol·L-1 d、0.08 mol·L-1
(4)在第5分钟末将容器的体积缩小一半后,若在第8分钟末达到新的平衡(此时NH3的浓度约为0.25 mol·L-1),请在上图中画出第5分钟末到此平衡时NH3浓度的变化曲线.
(5)如果上述反应在相同温度和容器中进行,欲使反应达到平衡时NH3的物质的量分数与原平衡相等,起始加入的三种物质的物质的量n(N2)、n(H2)、n(NH3)分别为a、b、c,则a、b、c之间应该满足的关系式为:
(6)若该反应在298K、398K时的化学平衡常数分别为K1、K2,则K1 K2(填“>” “="”" 或 “<” )。