网址:http://m.1010jiajiao.com/timu3_id_151152[举报]
有一固体混合物X,可能含有氯化钠、碳酸钾、亚硝酸钠、硫酸铝、碱式碳酸镁、碳酸氢钠等物质。为探究X的成分,设计方案并进行了如下实验:
I.将X粉末充分加热,有气体产生。
Ⅱ.取一定量X粉末,加入足量的蒸馏水,粉末全部溶解,得到无色溶液。
Ⅲ.用铂丝蘸取少量Ⅱ中所得溶液,在火焰上灼烧,产生黄色火焰;透过蓝色钴玻璃观察,无紫色火焰。
Ⅳ.取Ⅱ中无色溶液,滴人KMnO4酸性溶液,紫红色不褪去。
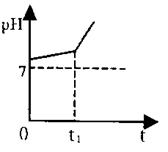
V.取Ⅱ中溶液,用惰性电极电解,开始时阳极产生的气体能使
湿润的淀粉碘化钾试纸变蓝色。电解过程中测得溶液pH变化如
右图所示。
(1)根据实验Ⅳ可判断,一定不存在的物质是_________ 。
(2)X的成分是_________ (填化学式)。
(3)实验V中,0~t时间内,阴极的电极反应式为:_________电池总反应的离子方程式_________
(4)0→t1时间内,溶液pH升高比较缓慢的原因是:_________ (用化学方程式表示)。

查看习题详情和答案>>
 有一固体混合物X,可能含有氯化钠、碳酸钾、亚硝酸钠、硫酸铝、碱式碳酸镁、碳酸氢钠等物质.为探究X的成分,设计方案并进行了如下实验:
有一固体混合物X,可能含有氯化钠、碳酸钾、亚硝酸钠、硫酸铝、碱式碳酸镁、碳酸氢钠等物质.为探究X的成分,设计方案并进行了如下实验:
I.将X粉末充分加热,有气体产生.
Ⅱ.取一定量X粉末,加入足量的蒸馏水,粉末全部溶解,得到无色溶液.
Ⅲ.用铂丝蘸取少量Ⅱ中所得溶液,在火焰上灼烧,产生黄色火焰;透过蓝色钴玻璃观察,无紫色火焰.
Ⅳ,取Ⅱ中无色溶液,滴人KMnO4酸性溶液,紫红色不褪去.
V.取Ⅱ中溶液,用惰性电极电解,开始时阳极产生的气体能使湿润的淀粉碘化钾试纸变蓝色.电解过程中测得溶液pH变化如
图所示.
(1)根据实验Ⅳ可判断,一定不存在的物质是______.
(2)X的成分是______(填化学式).
(3)实验V中,0~t时间内,两个电极上的电极反应式为:阳极______;阴极______.
(4)0→t1时间内,溶液pH升高比较缓慢的原因是:______(用离子方程式表示).
查看习题详情和答案>>
为探究X的成分,设计方案并进行了如下实验:
Ⅰ.将X粉末充分加热,有气体产生。
Ⅱ.取一定量X粉末,加入足量的蒸馏水,粉末全部溶解,得到无色溶液。
Ⅲ.用铂丝蘸取少量Ⅱ中所得溶液,在火焰上灼烧,产生黄色火焰;透过蓝色钴玻璃观察,无紫色火焰。
Ⅳ.取Ⅱ中无色溶液,滴入KMnO4酸性溶液,紫红色不褪去。
Ⅴ.取Ⅱ中溶液,用惰性电极电解,开始时阳极产生的气体能使湿润的淀粉碘化钾试纸变蓝色。电解过程中测得溶液pH变化如图所示。

(2)X的成分是____________(填化学式)。
(3)实验V中,0→t1时间内,两个电极上的电极反应式为:阳极______________;阴极_____________。
(4)0→t1时间内,溶液pH升高比较缓慢的原因是_______________(用离子方程式表示)。
有一固体混合物X,可能含有氯化钠、碳酸钾、亚硝酸钠、硫酸铝、碱式碳酸镁、碳酸氢钠等物质。为探究X的成分,设计方案并进行了如下实验:
I.将X粉末充分加热,有气体产生。
Ⅱ.取一定量X粉末,加入足量的蒸馏水,粉末全部溶解,得到无色溶液。
Ⅲ.用铂丝蘸取少量Ⅱ中所得溶液,在火焰上灼烧,产生黄色火焰;透过蓝色钴玻璃观察,无紫色火焰。
Ⅳ.取Ⅱ中无色溶液,滴人KMnO4酸性溶液,紫红色不褪去。
V.取Ⅱ中溶液,用惰性电极电解,开始时阳极产生的气体能使
湿润的淀粉碘化钾试纸变蓝色。电解过程中测得溶液pH变化如
右图所示。
(1)根据实验Ⅳ可判断,一定不存在的物质是_________ 。
(2)X的成分是_________ (填化学式)。
(3)实验V中,0~t时间内,阴极的电极反应式为:_________电池总反应的离子方程式_________
(4)0→t1时间内,溶液pH升高比较缓慢的原因是:_________ (用化学方程式表示)。
查看习题详情和答案>>
有一固体混合物X,可能含有氯化钠、碳酸钾、亚硝酸钠、硫酸铝、碱式碳酸镁、碳酸氢钠等物质。为探究X的成分,设计方案并进行了如下实验:
I.将X粉末充分加热,有气体产生。
Ⅱ.取一定量X粉末,加入足量的蒸馏水,粉末全部溶解, 得到无色溶液。
得到无色溶液。
Ⅲ.用铂丝蘸取少量Ⅱ中所得溶液,在火焰上灼烧,产生黄色火焰;透过蓝色钴玻璃观察,无紫色火焰。
Ⅳ.取Ⅱ中无色溶液,滴人KMnO4酸性溶液,紫红色不褪去。
V.取Ⅱ中溶液,用惰性电极电解,开始时阳极产生的气体能使
湿润的淀粉碘化钾试纸变蓝色。电解过程中测得溶液pH变化如
右图所示。
(1)根据实验Ⅳ可判断,一定不存在的物质是_________ 。
(2)X的成分是_________ (填化学式)。
(3)实验V中,0~t时间内,阴极的电极反应式为:_________电池总反应的离子方程式_________
(4)0→t1时间内,溶液pH升高比较缓慢的原因是:_________ (用化学方程式表示)。