��Ŀ����
��ͼ��ʾ������Ϊm��Сľ��A����������ΪM��ľ��B����ˣ�B��ˮƽ��������������ˮƽ�������������˶�����A��B��Ծ�ֹ��ijʱ�̳�ȥˮƽ����������һ��ʱ�䣬B�ڵ����ϻ�����һ�ξ���s��A��B�������B���һ�����һ�ξ���L��A��B��ͣ�¡���֪ľ��B�㹻����A��B��Ķ�Ħ������Ϊ��1��B������Ķ�Ħ������Ϊ��2���Ҧ�1����2����ȥˮƽ������![]()
��1��Сľ��A��ľ��B���Եļ��ٶȣ�
��2��Сľ��A��ľ��B����ʱ��֮�ȣ�
��3��ľ��B�ƶ�����s�ı���ʽ��
�⣺��1����ľ��A F1=��1mg=ma1
a1=��
��ľ��B F2=��2(M+m)g-��1mg=Ma2
a2=![]()
��2��t1=![]()
t2=![]()
![]() ��
��
��3����ľ��A
��1mg(L+s)= ![]() mv02
mv02
��ľ��B
�ۦ�2(M+m)g-��1mg��s=![]() Mv02
Mv02
s=![]() ��
��

��ϰ��ϵ�д�
�����Ŀ
 ��ͼ��ʾ������ΪM��Ш����龲ֹ��ˮƽ�����ϣ���б������Ϊ�ȣ�б������һ����Ϊm��С��飬С�����б��֮�����Ħ�����ú���F��б����������ʹ֮�����ϻ�����С����˶��Ĺ����У�Ш�����ʼ�ձ��־�ֹ��������
��ͼ��ʾ������ΪM��Ш����龲ֹ��ˮƽ�����ϣ���б������Ϊ�ȣ�б������һ����Ϊm��С��飬С�����б��֮�����Ħ�����ú���F��б����������ʹ֮�����ϻ�����С����˶��Ĺ����У�Ш�����ʼ�ձ��־�ֹ��������| A�������Ш������֧����Ϊ��M+m��g | B�������Ш������Ħ����Ϊ�� | C��Ш������С���Ħ��������Ϊ�� | D��С���һ���ܵ��ĸ������� |
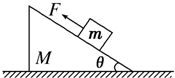
 ��ͼ��ʾ������ΪM��б�������ˮƽ���ϣ�����������Ϊm��С��飬���Ӵ������Ħ��������һ�ν�ˮƽ��F1����M�ϣ��ڶ��ν�F2����m�ϣ����ζ�Ҫ��m��M��������Ի�������F1��F2�ı�Ϊ��������
��ͼ��ʾ������ΪM��б�������ˮƽ���ϣ�����������Ϊm��С��飬���Ӵ������Ħ��������һ�ν�ˮƽ��F1����M�ϣ��ڶ��ν�F2����m�ϣ����ζ�Ҫ��m��M��������Ի�������F1��F2�ı�Ϊ�������� ��ͼ��ʾ������Ϊm��С��ˮƽ���Ϊ2mʱ���ٶȵĴ�СΪ4m/s��������ֱ���£�������˶��п��������Ĵ�С����������0.1��������������Ĺ����в���ʧ��е�ܣ���
��ͼ��ʾ������Ϊm��С��ˮƽ���Ϊ2mʱ���ٶȵĴ�СΪ4m/s��������ֱ���£�������˶��п��������Ĵ�С����������0.1��������������Ĺ����в���ʧ��е�ܣ��� ��ͼ��ʾ������Ϊm��С��A���ɾ�ֹ��ʼ�������䣬���ٶȴ�СΪ
��ͼ��ʾ������Ϊm��С��A���ɾ�ֹ��ʼ�������䣬���ٶȴ�СΪ ��ͼ��ʾ������ΪM����ͨ�������ֽ�����Ϊm�������Լ��ٶ�a���ᣬ�����������̣��˱��־�ֹ����������ֱ����н�Ϊ�ȣ���
��ͼ��ʾ������ΪM����ͨ�������ֽ�����Ϊm�������Լ��ٶ�a���ᣬ�����������̣��˱��־�ֹ����������ֱ����н�Ϊ�ȣ���