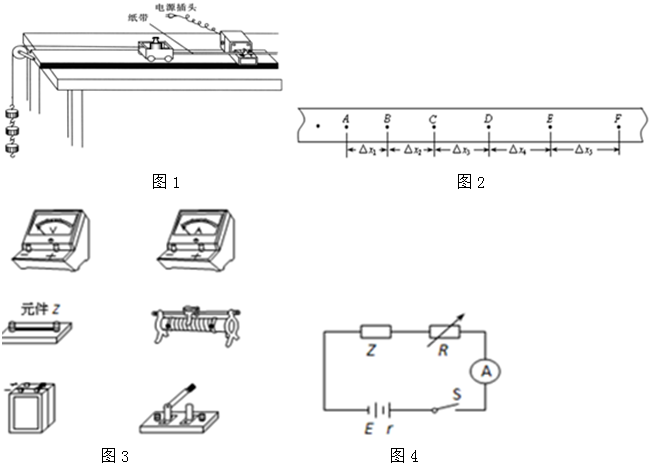
��Ŀ����
A���Ѵ���ʱ���̶��ڳ�ľ���һ�ˣ��Ӻõ�·
B����С��ͣ�ڿ�������ʱ��������ͨ��Դ���ͷ�С��
C������ֽ�����ظ�3��ʵ�飬ѡ��һ���������ֽ��
D����һ��ϸ��ϵ��С���ϣ�ϸ����������֣����������룬��ֽ����������ʱ������������һ�˹̶���С������
E���Ͽ���Դ��ȡ��ֽ��
������ʵ��˳��ӦΪ____��

 ������ҵ��ͬ����ϰ��ϵ�д�
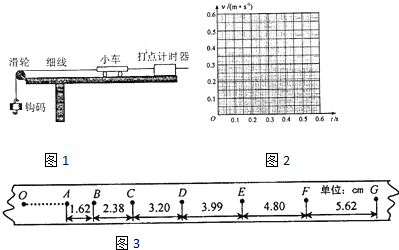
������ҵ��ͬ����ϰ��ϵ�д�1����8�֣�Ϊ��̽���ܵ���������ʱ�������˶��ٶ���ʱ��ı仯���ɣ�ijͬѧ�����ˡ����ٶ�����������������������ϵ����ʵ��װ��(��ͼ��)��ʵ��ʱ��ƽ��С����ľ��֮���Ħ��������С���ϰ�װһ���壬�����������С���˶���������

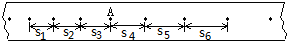
�����������м���һС���룬���ͷ�С��________(ѡ�֮ǰ����֮��)��ͨ����ʱ���ĵ�Դ����ֽ���ϴ��һϵ�еĵ㣮
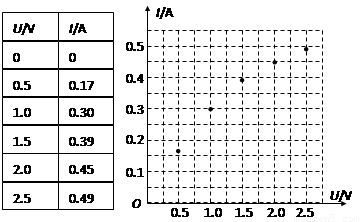
�ڴ�ֽ����ѡȡ���ɼ�������в������ó����������ʱ��t���ٶ�v���������±���
|
ʱ��t/s |
0 |
0.50 |
1.00 |
1.50 |
2.00 |
2.50 |
|
�ٶ�v/ (m��s��1) |
0.12 |
0.19 |
0.23 |
0.26 |
0.28 |
0.29 |
�����ʵ����������С����v��tͼ��

ͼ��
��ͨ����ʵ�����ķ�������ͬѧ��Ϊ�������˶��ٶȵ����ӣ�С�����ܵĿ���������������Ƿ�ͬ�����Ĺ۵㣿�����v��tͼ���Ҫ�������ɣ�
��2����10�֣��ڡ����С���ݵķ����������ߡ�ʵ���У�Ҫ����һ�����С�2.7V 1.5W���ĵ������˵ĵ�ѹ��ͨ�����ĵ����������������ģ�
Aֱ����Դ3V������ɲ��ƣ� Bֱ�����0��3A������Լ0.1����
Cֱ�����0��0.6A������Լ0.5���� Dֱ����ѹ��0��3V������Լ3k����
Eֱ����ѹ��0��15V������Լ200k���� F������������10����1A��
G������������1k����300mA��
�ٳ����ء������⣬Ϊ���ʵ�飬��Ҫ������������ѡ�� ������ĸ��
��ij��λͬѧ���������A��B��C��D��ʾ�ĵ�·ͼ����ȷ���� ����������ȷ�ĵ�·ͼ��ʵ��ͼ����ȷ�����ߡ�


���±��еĸ������ݸ�ͬѧ��ʵ���в�õģ����ݱ����е�������ͼ��ʾ�ķ���ֽ���Ѿ���õ㣬�������õ��ݵķ����������ߣ����ݸ����߿����жϣ����ѹ�������ݵĵ��� ��
 ��̽��С���ٶ���ʱ��仯���ɵ�ʵ���У�
��̽��С���ٶ���ʱ��仯���ɵ�ʵ���У�