��Ŀ����
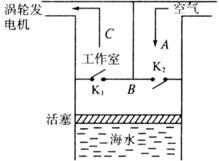
ģ��3-3����1964���Ƴ��������ϵ�һյ�ú��˷���ĺ���ƣ���������ʾ��ͼ��ͼ��ʾ�����ú������������������������A����������B��ѹ���������빤����C���ƶ����ֻ�������������硣����ˮ�½�ʱ������K1�رգ�K2������ˮ����ʱ��K2�رգ���ˮ�ƶ���������ѹ����������������Ϊ�������壩������ѹǿ�ﵽ6��105 Paʱ������K1�Ŵ�K1���������ƶ�B�еĿ�����ֱ������ȫ�������빤����CΪֹ��ͬʱ������C�Ŀ����ƶ����ֻ�������
��1������������Ϣ���ж�����˵������ȷ�Ĵ̡�������Ĵ���
�� ��A��ѹ������������B�п���������һ������
�� ��B���ڻ��������ƶ���K1δ��֮ǰ��B�еĿ�������Χ�ų�����
�� ��C���ڻ��������ƶ���K1δ��֮ǰ��B�е�ÿ���������Ӷ����ڵ�ײ��������
�� ��D��ѹ�����壬�����С������֮���ƽ�������С������ѹǿ������������Ϊ����
�� ��E���ú��˷�����ܽ����˵Ļ�е����ȫת��Ϊ����
��2����ÿ������ѹǿΪ1.0��105 Pa���¶�Ϊ7 ��Ŀ���0.336 m3���ڱ�״̬�µ����Ϊ���٣�ÿ������Ŀ�����������������Ӹ���Ϊ���٣���NA��6.0��1023/mol��
��1��������A ���̣�B ������C ������D ������E
��������1������������Ϊ�������壬����ֻ���¶Ⱦ�����������������ѹ��������B�п��������ܲ��䣬ѡ��A����ѹ�����������У���������������U=Q+W���������ܲ��䣬����K1δ��֮ǰ��B�еĿ�������Χ�ų�������ѡ��B��ȷ�����������ƶ���K1δ��֮ǰ��B�������ѹǿ���������Ӷ����ڵ�ƽ��ײ������������ÿһ����ѡ��C����������Ӽ����ϴ�ѹ�������������С������֮���ƽ�������С������ѹǿ������������Ϊ������ѡ��D�����������غ㣬�ú��˷���������ܽ����˵Ļ�е����ȫת��Ϊ���ܣ�ѡ��E����
��2����![]()
������֪p=p0 T0��273 K
V0=![]() m3=0.327 6 m3
m3=0.327 6 m3
���Ӹ���n=![]() ��6.0��1023��=8.775��1025����
��6.0��1023��=8.775��1025����

 ģ��3-5����
ģ��3-5����