��Ŀ����
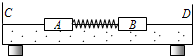 ���浼���dz��õ�һ��ʵ��������������������ʹ���ĵ����뻬��֮���γ����棬ʹ���������ڵ����ϣ������ڵ����ϵ��˶�����Ϊû��Ħ�������ǿ����ô���ֱ����C��D�����浼��ͻ���A��B��֤�����غ㶨�ɣ�ʵ��װ����ͼ��ʾ�����õ�ʵ�鲽�����£�
���浼���dz��õ�һ��ʵ��������������������ʹ���ĵ����뻬��֮���γ����棬ʹ���������ڵ����ϣ������ڵ����ϵ��˶�����Ϊû��Ħ�������ǿ����ô���ֱ����C��D�����浼��ͻ���A��B��֤�����غ㶨�ɣ�ʵ��װ����ͼ��ʾ�����õ�ʵ�鲽�����£�a���ɿ��ֵ�ͬʱ����¼����A��B�˶�ʱ��ļ�ʱ����ʼ��������A��B����ֱ���ײC��D����ʱ��ʱ��������ʱ������A��B�ֱ�C��D���˶�ʱ��t1��t2��
b����A��B��ˮƽ����һ���ᵯ�ɣ�����ѹסA��Bʹ����ѹ�������������浼���ϣ���������ֹ��ij��λ�ã�
c���������������������浼�죬ʹ���촦��ˮƽ��
d���ÿ̶ȳ߲��A�������C��ľ���L1��B���Ҷ���D��ľ���L2
��1��ʵ�鲽���˳����
��2��ʵ���л���Ҫ�IJ���������
����Ҫ��������������
��3����������������ʵ�����ݣ���֤�����غ㶨�ɵı���ʽ��
��4��ʵ���е�����A��B������ճ���û��Dz�ճ���ã�
������
����������ʵ���ԭ��ȷ�����������������Լ������IJ��裬����ԭ���г������غ㶨�ɵı���ʽ��
����⣺��1��ʵ�������˳����c��b��d��a��
��2��3�����������浼����������ֱ���˶�������A��B���еľ����ʱ���������ֿ�ʱ���ٶȣ����ݶ����غ㶨�ɵã�mAvA=mBvB����vA=
��vB=
����mA
=mB
��֪����Ҫ����A��B������mA��mB���������������ƽ��
��4��ֻ��A��B�е�ijһ��ճ���ã������ѵ��ɵ��������ǽ�ȥ�����Сϵͳ����ճ���ã�����A��B����������C��D��ʹ��ʱ�����𰸾��п����ԣ���
�ʴ�Ϊ����1��c��b��d��a�� ��2����ƽ������A��B������mA��mB��
��3��mA
=mB
��4�������ǿ��ŵģ��ο����У�ֻ��A��B�е�ijһ��ճ���ã������ѵ��ɵ��������ǽ�ȥ�����Сϵͳ����ճ���ã�����A��B����������C��D��ʹ��ʱ���ȵȣ�
��2��3�����������浼����������ֱ���˶�������A��B���еľ����ʱ���������ֿ�ʱ���ٶȣ����ݶ����غ㶨�ɵã�mAvA=mBvB����vA=
| L1 |
| t1 |
| L2 |
| t2 |
| L1 |
| t 1 |
| L2 |
| t2 |
��4��ֻ��A��B�е�ijһ��ճ���ã������ѵ��ɵ��������ǽ�ȥ�����Сϵͳ����ճ���ã�����A��B����������C��D��ʹ��ʱ�����𰸾��п����ԣ���
�ʴ�Ϊ����1��c��b��d��a�� ��2����ƽ������A��B������mA��mB��
��3��mA
| L1 |
| t 1 |
| L2 |
| t2 |
��4�������ǿ��ŵģ��ο����У�ֻ��A��B�е�ijһ��ճ���ã������ѵ��ɵ��������ǽ�ȥ�����Сϵͳ����ճ���ã�����A��B����������C��D��ʹ��ʱ���ȵȣ�
�������������Ĺؼ�֪����֤�����غ㶨�ɵ�ʵ��ԭ����֪��A��B��ɵ�ϵͳ�����غ㣮

��ϰ��ϵ�д�
�����Ŀ
 ��2011?������ģ�⣩���浼���dz��õ�һ��ʵ��������������������ʹ���ĵ����뻬��֮���γ����棬ʹ���������ڵ����ϣ������ڵ����ϵ��˶�����Ϊû��Ħ�������ǿ����ô���ֱ����C��D�����浼���Լ�����A��B����֤�����غ㶨�ɣ�ʵ��װ����ͼ��ʾ�����ɵij��Ⱥ��Բ��ƣ������õ�ʵ�鲽�����£�
��2011?������ģ�⣩���浼���dz��õ�һ��ʵ��������������������ʹ���ĵ����뻬��֮���γ����棬ʹ���������ڵ����ϣ������ڵ����ϵ��˶�����Ϊû��Ħ�������ǿ����ô���ֱ����C��D�����浼���Լ�����A��B����֤�����غ㶨�ɣ�ʵ��װ����ͼ��ʾ�����ɵij��Ⱥ��Բ��ƣ������õ�ʵ�鲽�����£�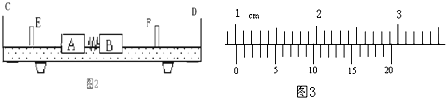
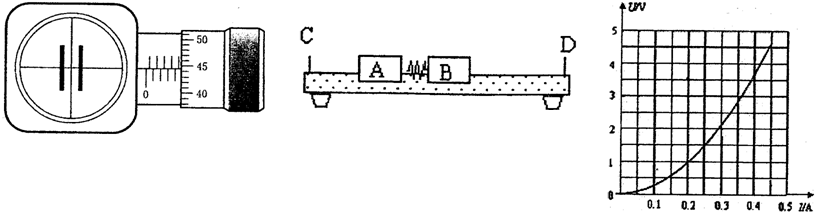
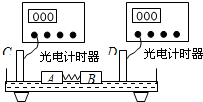 ���浼���dz��õ�һ��ʵ��������������������ʹ���ĵ����뻬��֮���γ����棬ʹ���������ڵ����ϣ������ڵ����ϵ��˶�����Ϊû��Ħ����ijͬѧ�ƻ���ͼʾװ������֤�����غ㶨�ɣ�Ԥ��ʵ�鲽�����£�
���浼���dz��õ�һ��ʵ��������������������ʹ���ĵ����뻬��֮���γ����棬ʹ���������ڵ����ϣ������ڵ����ϵ��˶�����Ϊû��Ħ����ijͬѧ�ƻ���ͼʾװ������֤�����غ㶨�ɣ�Ԥ��ʵ�鲽�����£�