��Ŀ����
B����ѡ��ģ��3-4��
��1������˵����ȷ����
A����������������֧���˹����˵�����ϸ���ʵ��������֧���˹�IJ���˵��
B���ӽ��յ��ĸ�Ƶ�ź��л�ԭ����Я����������ͼ���źŵĹ��̳�Ϊ���
C������Դ���߽���������ڽ����˶�ʱ�������ᷢ�ֲ���Ƶ�ʷ����˱仯����������ж�����ЧӦ��
D�����������ЧӦ��һ�����������ȷ����˶��ĸˣ��䳤���ܱȸ˾�ֹʱ�ij���С
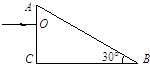
��2����ͼ��ʾ��Ϊ�ƹ⡢����ֱ�ͨ��ͬһ����װ���γɵĸ����������IJ��֣���ͼ��Ϊ �����ĸ������ƣ�ѡ��ƹ⡱�����⡱��������������ɫ�Ĺ���ͬ������������䵽�������ʵĽ����ϣ�ͼ��Ӧ��ɫ�ⷢ����ȫ���䣬��ͼ�Ҷ�Ӧ��ɫ�� ��ѡ�һ�����������ܡ������ܡ�������ȫ���䣮
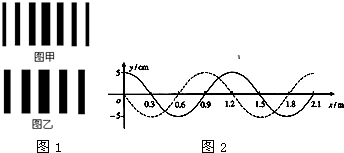
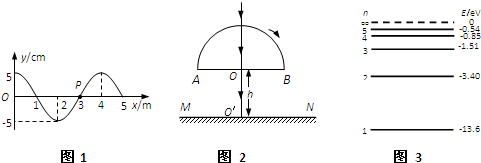
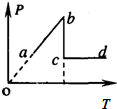
��3��ͼ��ʵ�ߺ����߷ֱ���x���ϴ�����һ�м�г�Შ��t=0��t=0.3sʱ�̵IJ���ͼ��x=1.2m�����ʵ���t=0.3sʱ����y���������˶�����
�ٲ��Ĵ�����������ڣ�
�ڲ��Ĵ������٣�
��1������˵����ȷ����
A����������������֧���˹����˵�����ϸ���ʵ��������֧���˹�IJ���˵��
B���ӽ��յ��ĸ�Ƶ�ź��л�ԭ����Я����������ͼ���źŵĹ��̳�Ϊ���
C������Դ���߽���������ڽ����˶�ʱ�������ᷢ�ֲ���Ƶ�ʷ����˱仯����������ж�����ЧӦ��
D�����������ЧӦ��һ�����������ȷ����˶��ĸˣ��䳤���ܱȸ˾�ֹʱ�ij���С
��2����ͼ��ʾ��Ϊ�ƹ⡢����ֱ�ͨ��ͬһ����װ���γɵĸ����������IJ��֣���ͼ��Ϊ

��3��ͼ��ʵ�ߺ����߷ֱ���x���ϴ�����һ�м�г�Შ��t=0��t=0.3sʱ�̵IJ���ͼ��x=1.2m�����ʵ���t=0.3sʱ����y���������˶�����
�ٲ��Ĵ�����������ڣ�
�ڲ��Ĵ������٣�
��������1������Ӧ���ղ��������Dz�����������֤������в����ԣ�֪������ĸ������Դ���߽����߾��뷢���仯ʱ���Ų���������ЧӦ��֪������۳���ЧӦ��
��2��������ݸ������Ƶļ���벨�������ȣ��ƹ�IJ��������ⳤȥ������
��3������t=0.3sʱ����y���������˶��жϲ��Ĵ�������������ʱ�̵IJ��Σ��õ����ڵ�ͨ����ɲ��ٹ�ʽv=
���٣�
��2��������ݸ������Ƶļ���벨�������ȣ��ƹ�IJ��������ⳤȥ������
��3������t=0.3sʱ����y���������˶��жϲ��Ĵ�������������ʱ�̵IJ��Σ��õ����ڵ�ͨ����ɲ��ٹ�ʽv=
| �� |
| T |
����⣺��1��A�����������Dz�����������֤������в����ԣ��������ߺ����ϸ��涼������֧���˹�IJ���˵����A����
B������Ǵӽ��յ��ĸ�Ƶ�ź��л�ԭ����Я����������ͼ���źŵĹ��̣���B��ȷ��
C������Դ���߽������������Զ��ʱ�������ᷢ�ֲ���Ƶ�ʷ����˱仯����������ж�����ЧӦ�������߾���û�б仯�����ܲ���������ЧӦ����C����
D�����������ЧӦ����ЧӦ��֪�����������ȷ����˶��ĸˣ��䳤���ܱȸ˾�ֹʱ�ij���С����D��ȷ��
��ѡBD
��2���������Ƶļ���벨�������ȣ��ƹ�IJ��������ⳤ����֪ͼ��Ϊ��������ĸ������ƣ�
���ʶ�����������ʴ��ڶԻƹ�������ʣ����ٽ�ǹ�ʽsinC=
֪��������ٽ��С�ڻƹ���ٽ�ǣ�������������ɫ�Ĺ���ͬ������������䵽�������ʵĽ����ϣ���ͼ��Ӧ�����ⷢ����ȫ���䣬��ͼ�Ҷ�Ӧ�Ļƹ���ܷ���ȫ���䣮
��3��������x=1.2m�����ʵ���t=0.3sʱ����y���������˶������ν�����ƽ�ƣ���֪�����Ҵ�������ʱ���t=��n+
��T����n=0��1��2�����������
����ΪT=
=
s����n=0��1��2��������
��2������Ϊv=
=��4n+3��m/s��n=0��1��2��������Ϊn��������
�ʴ�Ϊ��
��1��BD ��2�����⣬����
��3���ٸò���x���������˶�������Ϊ
s����n=0��1��2��������
�ڲ���vΪ=��4n+3��m/s��n=0��1��2��
B������Ǵӽ��յ��ĸ�Ƶ�ź��л�ԭ����Я����������ͼ���źŵĹ��̣���B��ȷ��
C������Դ���߽������������Զ��ʱ�������ᷢ�ֲ���Ƶ�ʷ����˱仯����������ж�����ЧӦ�������߾���û�б仯�����ܲ���������ЧӦ����C����
D�����������ЧӦ����ЧӦ��֪�����������ȷ����˶��ĸˣ��䳤���ܱȸ˾�ֹʱ�ij���С����D��ȷ��
��ѡBD
��2���������Ƶļ���벨�������ȣ��ƹ�IJ��������ⳤ����֪ͼ��Ϊ��������ĸ������ƣ�
���ʶ�����������ʴ��ڶԻƹ�������ʣ����ٽ�ǹ�ʽsinC=
| 1 |
| n |
��3��������x=1.2m�����ʵ���t=0.3sʱ����y���������˶������ν�����ƽ�ƣ���֪�����Ҵ�������ʱ���t=��n+
| 3 |
| 4 |
����ΪT=
| 4��t |
| 4n+3 |
| 1.2 |
| 4n+3 |
��2������Ϊv=
| �� |
| T |
�ʴ�Ϊ��
��1��BD ��2�����⣬����
��3���ٸò���x���������˶�������Ϊ
| 1.2 |
| 4n+3 |
�ڲ���vΪ=��4n+3��m/s��n=0��1��2��
���������⿼��������ʱ�̵IJ����г�ͨ�������������������ѧ֪ʶ����������������Ǹ߿�������������֮һ��

��ϰ��ϵ�д�
�����Ŀ
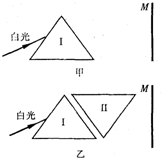 A����M���϶��·ֲ���ɫ��IJ�����С����
A����M���϶��·ֲ���ɫ��IJ�����С����