��Ŀ����
9��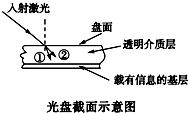 ����Ϣ����Ѹ�ͷ�չ�Ľ��죬�����Ǵ洢��Ϣ��һ����Ҫý�飮�����ϵ���Ϣͨ����ͨ������������ȡ�ģ������������Ǵ�ֱͶ�䵽�����ϣ��������ͨ�������ʲ�ʱ�ᷢ��ƫ�۶��ı��н��ķ�����ͼ��ʾ������˵������ȷ���ǣ�������
����Ϣ����Ѹ�ͷ�չ�Ľ��죬�����Ǵ洢��Ϣ��һ����Ҫý�飮�����ϵ���Ϣͨ����ͨ������������ȡ�ģ������������Ǵ�ֱͶ�䵽�����ϣ��������ͨ�������ʲ�ʱ�ᷢ��ƫ�۶��ı��н��ķ�����ͼ��ʾ������˵������ȷ���ǣ�������| A�� | ͼ�й������Ǻ�� �������� | |
| B�� | �ڹ��̵������ʲ��У������ڱȢٴ����ٶȸ��� | |
| C�� | �������٢��Ⱥ�ͨ��ͬһ��������װ�ã������ٵ��������ƱȢڵ�խ | |
| D�� | �������٢��Ⱥ�ͨ��ͬһ˫�����װ�ã������ٵ����ƿ��ȱȢڵĴ� |
���� ���ݹ��ƫ�۳̶ȱȽϳ�������������ʣ��Ӷ�����v=$\frac{c}{n}$�Ƚϳ��ڽ����д������ǵģ����ݲ����ij��̣��ж�˭��������������ԣ�
��� �⣺A���ٹ��ƫ�۳̶Ƚϴ��������ʽϴ�����������ʴ��ں��������ʣ����Ԣٹ������⣬���Ǻ�⣮��A����
B������v=$\frac{c}{n}$֪������������ʴ��ڽ����е��ٶ�С��������ڱȢٴ����ٶȸ��죮��B��ȷ��
C������������ʴ��ں��������ʣ�������IJ���С�ں��������ʲ������������Ƶļ���벨�������ȣ������ٹ⡢�ڹ��Ⱥ�ͨ��ͬһ˫�����װ�ã�ǰ�ߵõ������ƱȺ��ߵ�խ����C��ȷ��
D������������ʴ�Ƶ�ʴ�֪����IJ���С������Խ������������Խ���ԣ����Ԣٵõ����������ƱȢڵ���������խ����D����
��ѡ��BC
���� ��������ͻ�ƿ��DZȽϳ�������������ʴ�С���Ӷ��Ƚϳ�Ƶ�ʡ��������ڽ����е��ٶȡ��ٽ�ǵĴ�С��

��ϰ��ϵ�д�
�����Ŀ
20��һ��Ǧ���һ��Ƥ�����ѹ��ʱ������������ȷ���ǣ�������
| A�� | Ǧ���Ƥ���ѹ������Ƥ���Ǧ���ѹ�� | |
| B�� | Ƥ�������α��Ǧ��û�з����α� | |
| C�� | Ƥ���Ǧ���ѹ����Ǧ���Ƥ���ѹ��һ��ͬʱ���� | |
| D�� | Ǧ���Ƥ���ѹ����Ƥ���Ǧ���ѹ����һ��ƽ���� |
17����ͼ��ʾ��С��⻬б��Ӵ������߱����Ҵ�����ֱ������С���ܵ����������ǣ�������


| A�� | �������������� | B�� | ����������������б���֧���� | ||
| C�� | ������б��ĵ��� | D�� | �����������������»��� |
4����ͼ��ij�ʵ��˶����ٶ�ͼ����ͼ��õ�����ȷ����ǣ�������


| A�� | 0��2s�ڣ����ʵ��λ�ƴ�С��3 m | |
| B�� | 0��2s�ڣ����ʵ��ƽ���ٶ���1m/s | |
| C�� | ���ʵ���0��1s�����ܵ��ĺ�������2��4s�����ܵ��ĺ��� | |
| D�� | ���ʵ���0��1s�ڵ��˶�������2��4s�ڵ��˶������෴ |
18����һ�����ͽ�ͨ������ͼ���˿͵�������ʼ�ձ���ˮƽ�����˳���������ʱ���˿ͣ������� 

| A�� | ����ʧ��״̬ | B�� | ���ڳ���״̬ | ||
| C�� | �ܵ���ǰ��Ħ���� | D�� | �ܵ�����Ħ���� |
19������˵����ȷ���ǣ�������
| A�� | ������ٶȱ仯Խ������ٶ�Խ�� | |
| B�� | ���ɽǶȵ����������н�Խ�������ԽС | |
| C�� | Բ����ϵ���������������ܵ����������� | |
| D�� | ٤���Ե�����ʵ��˵����������ά�������˶���ԭ�� |
 ��ͼ��ʾ��ijͬѧ�ڵ���������һ������Ϊm=30kg����������ǰ������֪����������Ķ�Ħ������Ϊ��=0.2��б���ϵ�����F1��ˮƽ��н�Ϊ��=37�㣬��sin37��=0.6��cos37��=0.8��g=10m/s2�������������÷�����
��ͼ��ʾ��ijͬѧ�ڵ���������һ������Ϊm=30kg����������ǰ������֪����������Ķ�Ħ������Ϊ��=0.2��б���ϵ�����F1��ˮƽ��н�Ϊ��=37�㣬��sin37��=0.6��cos37��=0.8��g=10m/s2�������������÷����� ij̽��ѧϰС���ͬѧ������ͼװ���еĻ���Ϊ������֤�����ܶ�������������ʵ������װ��һ����ͼ��ʾ��װ�ã��������ǻ��ҵ��˴���ʱ�����õ�ѧ����Դ�����ߡ���дֽ��ֽ����Сľ�顢ϸɳ��������������ֽ������ϸ��ͨ�����ֹ��Ͽյ�СɳͰʱ���ͷ�СͰ�����鴦�ھ�ֹ״̬��������С���е�һλ��Ա��Ҫ��ɸ���ʵ�飬��
ij̽��ѧϰС���ͬѧ������ͼװ���еĻ���Ϊ������֤�����ܶ�������������ʵ������װ��һ����ͼ��ʾ��װ�ã��������ǻ��ҵ��˴���ʱ�����õ�ѧ����Դ�����ߡ���дֽ��ֽ����Сľ�顢ϸɳ��������������ֽ������ϸ��ͨ�����ֹ��Ͽյ�СɳͰʱ���ͷ�СͰ�����鴦�ھ�ֹ״̬��������С���е�һλ��Ա��Ҫ��ɸ���ʵ�飬��