��Ŀ����

��1���ڼס�����װ���У�С��a�»���������б�۹���������Ħ���������ʵ����
��2������ʵ��װ�ã�a��Ϊ����С��b��Ϊ����С���������оٵ���ʵ�����мס���װ��Ӧ�����������
A��ʵ��ʱ��Ҫ��������С��������ͱ���С�������
B����װʵ��װ��ʱ��װ�üױ���Ҫ�ش��ߣ���װ���ҿ��Բ���
C������С��ÿ�β��ش�б���ϵ�ͬһλ���ɾ�ֹ�ͷ�
D��б��ĩ�˵����߱���ˮƽ��С�����б��ĩ�˴���Ӧ�ܾ�ֹ
��3���ڼ�װ�õ�ʵ���¼�У�a����ǰ��ƽ���˶���ˮƽλ����ͼ�е�
��4����ͬѧʹ����װ�ã���������£�
���Ƚ�б�۹����ĩ�˵���ˮƽ����һ��ƽľ������Ⱥ��ϰ�ֽ��дֽ��������ľ����ֱ�����ڽ����ۿڴ���ʹС��a��б�۹����ij�̶��㴦�ɾ�ֹ�ͷţ�ײ��ľ�岢�ڰ�ֽ�����ºۼ�O��
�ڽ�ľ������ƽ���ʵ��ľ��룬��ʹС��a��ԭ�̶����ɾ�ֹ�ͷţ�ײ��ľ���ϲ��ڰ�ֽ�����ºۼ�B��
�۱���ľ�岻�����Ѱ뾶��ͬ��С��b��ֹ����б�۹��ˮƽ�ε����Ҷˣ���С��a�Դ�ԭ�̶����ɾ�ֹ�ͷţ���С��b����������ײ��ľ���ϲ��ڰ�ֽ�����ºۼ�A��C��
������ƽ����a��b��С��������ֱ�Ϊma��mb���ÿ̶ȳ߲�����ֽO�㵽A��B��C����ľ���ֱ�Ϊyl��y2��
y3������ͬѧ������ʵ��������õ�������֤������ײ���̶����غ㣬�����ʽΪ
| ma | ||
|
| ma | ||
|
| mb | ||
|
| ma | ||
|
| ma | ||
|
| mb | ||
|
��2��A����ΪҪ��֤��ײǰ����ܶ����Ƿ���ȣ��������С�����������A��ȷ��
B����װʵ��װ��ʱ����װ�ö���Ҫ�ش��ߣ���B����
C��Ϊ�˱�֤С��˵��ٶ���ͬ��С��ÿ���ɾ�ֹ��ͬһλ���ͷţ���C����
D��Ϊ�˱�֤С��ɳ�б����ƽ���˶�������б�۵�ĩ����ˮƽ��С�����б��ĩ��Ӧ�ܾ�ֹ����D��ȷ��
��ѡ��AD��
��3��a����b������b����ٶȴ���a����ٶȣ�����b����N�㣬a����M�㣬��Ϊa���ͷ�ʱƽ���˶����ٶȴ�������a����ٶȣ���֪a����ǰ��ƽ���˶�����P�㣮
��4��ƽ���˶���ˮƽλ�ƣ�����t=
|
| x | ||||
|
| x | ||||
|
| x | ||||
|
����֤��mava=mava��+mbvb��
����
| ma | ||
|
| ma | ||
|
| mb | ||
|
�ʴ�Ϊ����1�����
��2��AD��
��3��OP��ON��
��4��
| ma | ||
|
| ma | ||
|
| mb | ||
|
| 1 | ||
|

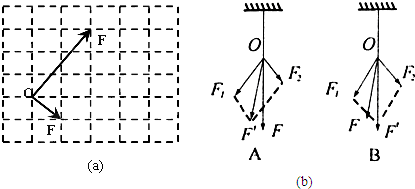
��1����12�֣�ijͬѧ������֤����ƽ���ı��ζ���ʵ�飬��Ҫ�������£�
A���������Ϸ�һ�鷽ľ�壬�ڷ�ľ������һ�Ű�ֽ����ͼ���Ѱ�ֽ���ڷ�ľ���ϣ�
B����ͼ������Ƥ����һ�˹̶��ڰ��ϵ�A�㣬����Ƥ������һ��˩������ϸ����ϸ������һ��ϵ�����ף�
C������ֻ���ɲ�����ͨ��ϸ�����ɽǶȵ�����Ƥ����ʹ������Ƥ���Ľ��ﵽijһλ��O������O���λ�ã������������ɲ����Ƶ�ʾ����
D����ѡ�õı�ȣ���Ǧ�ʺͿ̶ȳ������������ɲ����Ƶ�����F1��F2��ͼʾ������ƽ���ı��ζ�����������F�䣻
E����һ�����ɲ����ƣ�ͨ��ϸ��������Ƥ��ʹ���쳤���������ɲ����Ƶ�ʾ��������ϸ���ķ���ͬһ������������F��ͼʾ��
F���Ƚ���F����F�Ĵ�С�ͷ���
����������
������Ҫ��©���ݵIJ�������� �� ����©���ݷֱ��� �� __
����������©���ݲ��Ϻ���ͼ��a����ʾ��������������ֽ���µ���Ƥ����λ��O�㼰�����ɲ����������Ĵ�С������ͼ��a����������ʵ����������F1��F2�ĺ���ͼʾ������F���ʾ������
|
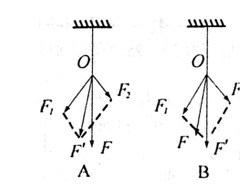 ��ͼ��b���Ǽ�����λͬѧ�ֱ�������ʵ��ʱ�õ��Ľ����������һ���ȽϷ���ʵ����ʵ������F��ֻ��һֻ���ɲ�������ʱ��ͼʾ��__________
��ͼ��b���Ǽ�����λͬѧ�ֱ�������ʵ��ʱ�õ��Ľ����������һ���ȽϷ���ʵ����ʵ������F��ֻ��һֻ���ɲ�������ʱ��ͼʾ��__________ 
(a) (b)

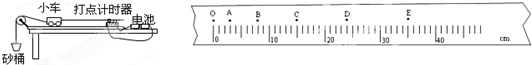
(2)��8�֣� ��ijͬѧ������ͼ��ʾװ��������֤ţ�ٵڶ����ɡ���ʵ�飬������ͼʾ״̬�¿�ʼ��ʵ�飬��ָ��ͼ�е���Ҫ�����ǣ�����д����� �� ��
�ڸ�ͬѧҪ̽��С���ļ��ٶ�a������M�Ĺ�ϵ��Ӧ�ñ��� ������ͬѧҪ̽�����ٶ�a������F��ϵ��Ӧ�ñ��� ���䣻
 ������ʵ���еõ�����ͼ��ʾֽ����ֽ���ϵļ�������A��B��C��D��E��ʾ������ͼ�����ݣ�С������B��ʱ���ٶ��� m/s�������ڼ�������ʱ����Ϊ0.1s����������������λ��Ч���֣�
������ʵ���еõ�����ͼ��ʾֽ����ֽ���ϵļ�������A��B��C��D��E��ʾ������ͼ�����ݣ�С������B��ʱ���ٶ��� m/s�������ڼ�������ʱ����Ϊ0.1s����������������λ��Ч���֣�
��1����12�֣�ijͬѧ������֤����ƽ���ı��ζ���ʵ�飬��Ҫ�������£�
A���������Ϸ�һ�鷽ľ�壬�ڷ�ľ������һ�Ű�ֽ����ͼ���Ѱ�ֽ���ڷ�ľ���ϣ�
B����ͼ������Ƥ����һ�˹̶��ڰ��ϵ�A�㣬����Ƥ������һ��˩������ϸ����ϸ������һ��ϵ�����ף�
C������ֻ���ɲ�����ͨ��ϸ�����ɽǶȵ�����Ƥ����ʹ������Ƥ���Ľ��ﵽijһλ��O������O���λ�ã������������ɲ����Ƶ�ʾ����
D����ѡ�õı�ȣ���Ǧ�ʺͿ̶ȳ������������ɲ����Ƶ�����F1��F2��ͼʾ������ƽ���ı��ζ�����������F�䣻
E����һ�����ɲ����ƣ�ͨ��ϸ��������Ƥ��ʹ���쳤���������ɲ����Ƶ�ʾ��������ϸ���ķ���ͬһ������������F��ͼʾ��
F���Ƚ���F����F�Ĵ�С�ͷ���
����������
������Ҫ��©���ݵIJ�������� �� ����©���ݷֱ��� �� __
����������©���ݲ��Ϻ���ͼ��a����ʾ��������������ֽ���µ���Ƥ����λ��O�㼰�����ɲ����������Ĵ�С������ͼ��a����������ʵ����������F1��F2�ĺ���ͼʾ������F���ʾ������
|
 ��ͼ��b���Ǽ�����λͬѧ�ֱ�������ʵ��ʱ�õ��Ľ����������һ���ȽϷ���ʵ����ʵ������F��ֻ��һֻ���ɲ�������ʱ��ͼʾ��__________
��ͼ��b���Ǽ�����λͬѧ�ֱ�������ʵ��ʱ�õ��Ľ����������һ���ȽϷ���ʵ����ʵ������F��ֻ��һֻ���ɲ�������ʱ��ͼʾ��__________

(a) (b)

(2)��8�֣� ��ijͬѧ������ͼ��ʾװ��������֤ţ�ٵڶ����ɡ���ʵ�飬������ͼʾ״̬�¿�ʼ��ʵ�飬��ָ��ͼ�е���Ҫ�����ǣ�����д����� �� ��
�ڸ�ͬѧҪ̽��С���ļ��ٶ�a������M�Ĺ�ϵ��Ӧ�ñ��� ������ͬѧҪ̽�����ٶ�a������F��ϵ��Ӧ�ñ��� ���䣻
 ������ʵ���еõ�����ͼ��ʾֽ����ֽ���ϵļ�������A��B��C��D��E��ʾ������ͼ�����ݣ�С������B��ʱ���ٶ���
m/s�������ڼ�������ʱ����Ϊ0.1s����������������λ��Ч���֣�
������ʵ���еõ�����ͼ��ʾֽ����ֽ���ϵļ�������A��B��C��D��E��ʾ������ͼ�����ݣ�С������B��ʱ���ٶ���
m/s�������ڼ�������ʱ����Ϊ0.1s����������������λ��Ч���֣�