��Ŀ����
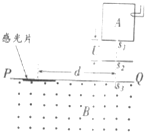
 ���������������������������ԭ��ʾ��ͼ���跨ʹij�л����������̬���ӵ���ͼ����ʾ�������У�ʹ���ܵ������������ʧȥһ�����ӱ��Ϊ��һ�۵ķ������ӣ��������Ӵ�����s1�Ժ�С���ٶȽ����ѹΪU�ļ��ٵ糡�������ٲ��ƣ������ٺ���ͨ������s2��s3����Ÿ�ǿ��ΪB����ǿ�ų�������ֱ�ڴų����Ľ���PQ����������Ӵй�Ƭ�ϣ��γɴ�ֱ��ֽ����ƽ��������s3��ϸ�ߣ������ϸ�ߵ�����s3�ľ���Ϊd��
���������������������������ԭ��ʾ��ͼ���跨ʹij�л����������̬���ӵ���ͼ����ʾ�������У�ʹ���ܵ������������ʧȥһ�����ӱ��Ϊ��һ�۵ķ������ӣ��������Ӵ�����s1�Ժ�С���ٶȽ����ѹΪU�ļ��ٵ糡�������ٲ��ƣ������ٺ���ͨ������s2��s3����Ÿ�ǿ��ΪB����ǿ�ų�������ֱ�ڴų����Ľ���PQ����������Ӵй�Ƭ�ϣ��γɴ�ֱ��ֽ����ƽ��������s3��ϸ�ߣ������ϸ�ߵ�����s3�ľ���Ϊd����1�������������ӵ�����m�ı���ʽ
��2�����ݷ������ӵ�������M�����Ʋ��л�������Ľṹ��ʽ����ij�ֺ�C��H��±�صĻ������M
Ϊ48��д����ṹ��ʽ
��3������ij�ֺ�C��H��±�صĻ�����������Mֵ���ֱ�Ϊ64��66����˵��ԭ��д�����ǵĽṹ��ʽ��
���Ʋ��л�������Ľṹʱ�������õ��ĺ����϶��ͬλ�ص����������±���
| Ԫ �� | H | C | F | Cl | Br |
| �����϶��ͬλ���������� | 1 | 12 | 19 | 35��37 | 79��81 |
��������1�������ɹ��ܹ�ϵ�ó����ӵ��ٶȣ�����ų�������������������������Բ���˶�����й�Ƭ�������ľ���ӦΪһ��ֱ���ľ��룮�ɴ˿��Խ��������
��2������Ǵ���ѧ������������Ϊ48���ʻ������±��Ӧ����F���ɴ˿���д����ʽ
��3���ۺ�����������֪��±���е�Cl���ɴ˿���ȷ������ʽ��
��2������Ǵ���ѧ������������Ϊ48���ʻ������±��Ӧ����F���ɴ˿���д����ʽ
��3���ۺ�����������֪��±���е�Cl���ɴ˿���ȷ������ʽ��
����⣺
��1����m��q��ʾ���ӵ���������������v��ʾ���Ӵ�����s2���ʱ���ٶȣ��ɹ��ܹ�ϵ�ɵ�
qU=
mv2 ��
����ų���������������������Բ���˶�����ţ�ٶ��ɿɵ�
qvB=m
��
ʽ��RΪԲ�İ뾶��
�й�Ƭ�ϵ�ϸ���ߵ�s3��ľ���d=2R ��
�⣺m=
��
��2��CH3CH2F
��3����M����ֵ�жϸû����ﲻ���ܺ�Br��ֻ���ܺ�Cl������ΪCl��������ͬλ�أ���
35Cl��37Cl�����Բ�����躬C��H��±�ص�ij�л�������������Mֵ�����Ӧ�ķ��ӽṹ��
ʽΪ��CH3CH235Cl M=64��
CH3CH237Cl M=66
��
��1�����ӷ�������Ϊ��m=
��2��������ṹ��ʽΪ��CH3CH2F
��3���ṹ��ʽ�ֱ�Ϊ��CH3CH235Cl CH3CH237Cl
��1����m��q��ʾ���ӵ���������������v��ʾ���Ӵ�����s2���ʱ���ٶȣ��ɹ��ܹ�ϵ�ɵ�
qU=
| 1 |
| 2 |
����ų���������������������Բ���˶�����ţ�ٶ��ɿɵ�
qvB=m
| v2 |
| B |
ʽ��RΪԲ�İ뾶��
�й�Ƭ�ϵ�ϸ���ߵ�s3��ľ���d=2R ��
�⣺m=
| gB2d2 |
| 8U |
��2��CH3CH2F
��3����M����ֵ�жϸû����ﲻ���ܺ�Br��ֻ���ܺ�Cl������ΪCl��������ͬλ�أ���
35Cl��37Cl�����Բ�����躬C��H��±�ص�ij�л�������������Mֵ�����Ӧ�ķ��ӽṹ��
ʽΪ��CH3CH235Cl M=64��
CH3CH237Cl M=66
��
��1�����ӷ�������Ϊ��m=
| gB2d2 |
| 8U |
��2��������ṹ��ʽΪ��CH3CH2F
��3���ṹ��ʽ�ֱ�Ϊ��CH3CH235Cl CH3CH237Cl
���������⻯ѧԪ�ض�������Ԫ�أ�����һ�ʣ������Ǵ���ѧ֪ʶ��

��ϰ��ϵ�д�
�����Ŀ
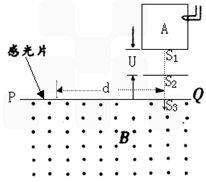
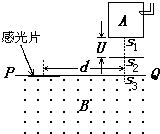 ��ͼ�Dz�������������������������ԭ��ʾ��ͼ���跨ʹij�л����������̬���ӵ���ͼ����ʾ������A�У�ʹ���ܵ������������ʧȥһ�����ӱ��Ϊ��һ�۵ķ������ӣ��������Ӵ�����s1�Ժ�С���ٶȽ����ѹΪ U�ļ��ٵ糡�������ٲ��ƣ������ٺ���ͨ������s2��s3����Ÿ�ǿ��Ϊ B����ǿ�ų�������ֱ�ڴų����Ľ��� PQ����������Ӵй�Ƭ�ϣ��γɴ�ֱ��ֽ����ƽ��������s3��ϸ�ߣ������ϸ�ߵ�����s3�ľ���Ϊd��
��ͼ�Dz�������������������������ԭ��ʾ��ͼ���跨ʹij�л����������̬���ӵ���ͼ����ʾ������A�У�ʹ���ܵ������������ʧȥһ�����ӱ��Ϊ��һ�۵ķ������ӣ��������Ӵ�����s1�Ժ�С���ٶȽ����ѹΪ U�ļ��ٵ糡�������ٲ��ƣ������ٺ���ͨ������s2��s3����Ÿ�ǿ��Ϊ B����ǿ�ų�������ֱ�ڴų����Ľ��� PQ����������Ӵй�Ƭ�ϣ��γɴ�ֱ��ֽ����ƽ��������s3��ϸ�ߣ������ϸ�ߵ�����s3�ľ���Ϊd��