��Ŀ����
��������÷���ԭ����������Ҫ�������乤�ߣ���1���ҹ�����3�ż������ɽΣ�ͨ�������������������õķ�����ԼΪ�����������2�����ң����������ո���ɺ���ֱ������ijʱ�̣�������ٶȴ�СΪv���ڴ˺�һ���϶̵�ʱ��t�ڣ��������������������Ϊ��m���������������ڵ�����ٶȷ������£���СΪu������һ��ʱ���ڻ���ܵ��ķ�������С��������ʱ������������������С������Ϊ�˶�ʱ���ڻ���ܵ��ķ�������С���䣩
��2������е����Ͷ༶֮�֣��༶������ǰѻ��һ��һ���ؽ���һ�𣬵�һ��ȼ������֮��Ѽ������������Ḻ����Ȼ��ڶ�����ʼ������ȼ������֮���ٰѵڶ�������������˴������Ͻ����༶����ܱȵ��������ø�����ٶȣ�
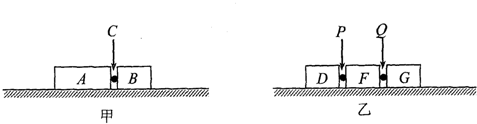
ijͬѧ�ֱ���������������ѧģ�������Ե�ģ�ⵥ������Ͷ��������ˮƽ����ʱ�Ĺ������̣���ͼ�ס�����ʾ����ͼ�еĹ⻬ˮƽ���ϲ��ž�ֹ�����������ֱ�Ϊ2m��m���������A��B������֮��ճ������ըҩC����ըʱ�ͷų�������Ϊ2��E����ͼ�й⻬ˮƽ���ϲ��ž�ֹ������������Ϊm���������D��F��G��D��F֮���F��G֮��ֱ�ճ������ըҩP��Q��ͨ������ʹըҩQ�ȱ�ը��ըҩP��ը��Q��P��ըʱ�ͷų���������Ϊ��E������ըҩ�����������Բ��ƣ���ը��ʱ�伫�̣���ը������������ת��Ϊ��е�ܣ���ը�����������ٶȷ�����ͬһֱ���ϣ������еı�ը�����������D���ٶ������A���ٶȵı�����

���𰸡����������ö���������ⷴ������С��
���ݶ����غ㶨���г���ʽ������⣮
ͬһ��������Ҫѡ��ͬ��ϵͳ��Ϊ�о�����
���������غ�������⣮
����⣺�跢�����������������ΪF����tʱ�����������ö��������ã�
Ft=��mu-��-��mv�� ��
��ţ�ٵ������ɵã�
����ܵ��ķ�������СF��=F ��
�ɢ٢ڵã�
F��=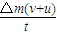 ��
��
��2���ڼ�ͼ���ж����غ�ã�
2mvA=mvB ��
�������غ�ã�
2��E= 2mvA2+
2mvA2+ mvB2 ��
mvB2 ��
�ɢܢݵã�
vA=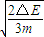 ��
��
����ͼ��ըҩ��ը�������غ�ã�
2mvDF=mvG ��
�������غ�ã�
��E= 2mvDF2+
2mvDF2+ mvG2 ��
mvG2 ��
ըҩ��ըʱ��
2mvDF=mvD+mvF ��
�������غ�ã�
 2mvDF2+��E=
2mvDF2+��E= mvD2+
mvD2+ mvF2 ��
mvF2 ��
�ɢߢ���ã�
vD= ��1+
��1+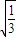 ��
��
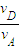 =
=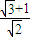
�����еı�ը�����������D���ٶ������A���ٶȵ� ��
��
�����������غ�Ͷ���������Ӧ�ö�Ҫע�ⷽ��
���ڶ�����ʸ�������з����ԣ�Ϊ�����ȹ涨һ��������Ȼ���ڴ˻����Ͻ����о���
�Ѷ����غ�������غ��������г���ʽ����dz��������⣮
���ݶ����غ㶨���г���ʽ������⣮
ͬһ��������Ҫѡ��ͬ��ϵͳ��Ϊ�о�����
���������غ�������⣮
����⣺�跢�����������������ΪF����tʱ�����������ö��������ã�
Ft=��mu-��-��mv�� ��
��ţ�ٵ������ɵã�
����ܵ��ķ�������СF��=F ��
�ɢ٢ڵã�
F��=
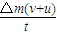 ��
����2���ڼ�ͼ���ж����غ�ã�
2mvA=mvB ��
�������غ�ã�
2��E=
 2mvA2+
2mvA2+ mvB2 ��
mvB2 ���ɢܢݵã�
vA=
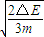 ��
������ͼ��ըҩ��ը�������غ�ã�
2mvDF=mvG ��
�������غ�ã�
��E=
 2mvDF2+
2mvDF2+ mvG2 ��
mvG2 ��ըҩ��ըʱ��
2mvDF=mvD+mvF ��
�������غ�ã�
 2mvDF2+��E=
2mvDF2+��E= mvD2+
mvD2+ mvF2 ��
mvF2 ���ɢߢ���ã�
vD=
 ��1+
��1+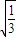 ��
��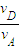 =
=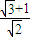
�����еı�ը�����������D���ٶ������A���ٶȵ�
 ��
�������������غ�Ͷ���������Ӧ�ö�Ҫע�ⷽ��
���ڶ�����ʸ�������з����ԣ�Ϊ�����ȹ涨һ��������Ȼ���ڴ˻����Ͻ����о���
�Ѷ����غ�������غ��������г���ʽ����dz��������⣮

��ϰ��ϵ�д�
�����Ŀ