��Ŀ����
 ��ͼ��ʾΪһ�ֲⶨ�˶�Ա���ܵ�װ�ã��˶�Ա������Ϊm1������һ��˩�����䲢��ˮƽ���������֣����ƻ���������Ħ�����������¶�����һ������Ϊm2������������Ŵ��ʹ����˵����IJ�����ʹ���ʹ�������v���������˶�������˵������ȷ���ǣ�������
��ͼ��ʾΪһ�ֲⶨ�˶�Ա���ܵ�װ�ã��˶�Ա������Ϊm1������һ��˩�����䲢��ˮƽ���������֣����ƻ���������Ħ�����������¶�����һ������Ϊm2������������Ŵ��ʹ����˵����IJ�����ʹ���ʹ�������v���������˶�������˵������ȷ���ǣ��������������������ˡ��˶Դ��ʹ���������������������������������ؼ��������
����⣺A���˵����IJ��䣬��������û��λ�ƣ���A����
B�����ʹ��ܵ��˵�Ħ�������������ң����ʹ�������λ�ƣ����˶Դ��ʹ�����������B����
C���˶�ʱ��t���ʹ�λ��S=vt��Ħ����F=m2g�����˶�Ա����������ԼΪm2gvt����C��ȷ��D����
��ѡ��C
B�����ʹ��ܵ��˵�Ħ�������������ң����ʹ�������λ�ƣ����˶Դ��ʹ�����������B����
C���˶�ʱ��t���ʹ�λ��S=vt��Ħ����F=m2g�����˶�Ա����������ԼΪm2gvt����C��ȷ��D����
��ѡ��C
���������⿼���������������������ù��ܹ�ϵ���д�����Ŀ����⣮

��ϰ��ϵ�д�
 ��������ϵ�д�
��������ϵ�д� ����˼ά����ѵ����ʱ��ѧ��ϵ�д�
����˼ά����ѵ����ʱ��ѧ��ϵ�д�
�����Ŀ

 ������ⶨ�����ھ�d���������ھ���С�������α꿨��ֱ�Ӳ�������֪���ֽ����ĵ�����Ϊ
������ⶨ�����ھ�d���������ھ���С�������α꿨��ֱ�Ӳ�������֪���ֽ����ĵ�����Ϊ ��ʵ�����п����ṩ�������ģ�
��ʵ�����п����ṩ�������ģ�

 �ı���ʽΪ
�ı���ʽΪ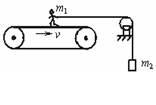 B���ڴ��ʹ����ʲ��������£����Ӵ����������������
B���ڴ��ʹ����ʲ��������£����Ӵ����������������  A���˶Դ��ʹ�����
A���˶Դ��ʹ�����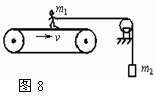 A���˶Դ��ʹ�����
A���˶Դ��ʹ�����