��Ŀ����
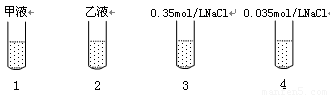
��12�֣�Ⱦ�϶��ȵ���ƣ�DCPIP��������̬������ɫ������ԭʱ�����ַۺ�ɫ����ɫ�����������ʵ�Ҷ����ƽ���ֳɼס���2�ݣ��ֱ�������������0.35mol/LNaCl��Һ��0.035mol/LNaCl��Һ��ҡ���Ƴ���Һ��Һ����Һ����������ʵ�飺
 |
��ȡ�ɾ����Թ�4֧�����1��2��3��4�ţ��ںڰ�������ͼ��ʾ�ֱ���������������Һ���Լ���
�ڽ���֧�Թ����ڹ���ǿ����ͬ�����˵Ĺ�Դ�£�����ÿ֧�Թ��м���2������ɫ�Ķ��ȵ������Һ��ҡ�ȣ��۲졣
��ʵ���������1���Թܲ������ݣ���Һ������ɫ��ɷۺ�ɫ�����ճ���ɫ��������֧�Թ������ݣ���Һ��������ɫ���ش����⣺
��1������ʵ������������
�� 1���Թ������е�����ɷ���__________________________��
�� 2���Թ�û�����������ԭ����________________________________________________
______________________________________________________________________________��
�� 1���Թ�����ɫ��ʧ��ԭ���ǣ�________________________________________________
______________________________________________________________________________��
��2����ʵ���ʵ��ԭ����
��Ⱦ�϶��ȵ���ƣ�DCPIP��������̬������ɫ������ԭʱ�����ַۺ�ɫ����ɫ������Ϊ __________________________________________________________________________��
��___________________________________________________________________________��
��____________________________________________________________________________��
��12�֣���1����������O2�� �� 0.035mol/LNaCl��Ũ�ȵ���Ҷ������ʣ���0.035mol/LNaCl�ǵ�����Һ����Ҷ����ͨ����������ˮ���ѣ����ṹ���ƻ��������ܽ��й�����ã��ⷴӦ��û������������
�ۣ�������ã��ⷴӦ�ֽ�ˮ������ԭ�⣨[H]��NADPH������ԭ�ԭ���ȵ����ʹ�䣨������̬ת��ɻ�ԭ̬��������ɫ��
��2���ٹ�����ò�����ԭ����ָʾ������Ҷ����ֻ�б��ֽṹ�������Բ��ܽ��й�����ã�Ҷ�����ǹ�����õĻ�����λ����
���ڵ�������Ũ�ȣ���Һ�У������壩Ҷ���巢����ͨ������������ˮ�����ѣ���
����

��.����aͼ��ʾ����Ҷ��ϸ����������Ҫ����������C��H��O�ı仯��bͼ��ʾһ����ֲ�з���ֲ����ܱ����������������ı仯���ߡ����ͼ�ش�
 |
��1��aͼ�мס������������������ij����ֱ�Ϊ �� ������ ��
��2��aͼ�����У�B�� ������岿λ�������ģ��ҹ����У�A�����÷����ڸù��̵ĵ� �Ρ�
?����3��bͼ�з���ֲ���������ͷŵ�O2���ͺ����������ĵ�O2����ȵĵ��� ���÷���ֲ�꾭��һ��ҹ���Ƿ�������л�� ���� �������� ��
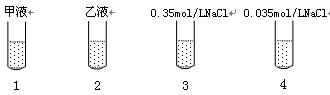
?����.Ⱦ�϶��ȵ���ƣ�DCPIP��������̬������ɫ������ԭʱ�����ַۺ�ɫ����ɫ�����������ʵ�Ҷ����ƽ���ֳɼס���2�ݣ��ֱ�������������0.35mol��L NaCl��Һ��0.035mol��L NaCl��Һ��ҡ���Ƴ���Һ��Һ����Һ����������ʵ�飺
 ��ȡ�ɾ����Թ�4֧�����1��2��3��4�ţ��ںڰ�������ͼ��ʾ�ֱ���������������Һ���Լ���
��ȡ�ɾ����Թ�4֧�����1��2��3��4�ţ��ںڰ�������ͼ��ʾ�ֱ���������������Һ���Լ����ڽ���֧�Թ����ڹ���ǿ����ͬ�����˵Ĺ�Դ�£�����ÿ֧�Թ��м���2������ɫ�Ķ��ȵ������Һ��ҡ�ȣ��۲졣
��ʵ���������1���Թܲ������ݣ���Һ������ɫ��ɷۺ�ɫ�����ճ���ɫ��������֧�Թ������ݣ���Һ��������ɫ���ش����⣺?����ʵ������������
��1���Թ������е�����ɷ��� ��
��2���Թ�û�����������ԭ���� ��
��1���Թ�����ɫ��ʧ��ԭ����������������������������������������
��.����aͼ��ʾ����Ҷ��ϸ����������Ҫ����������C��H��O�ı仯��bͼ��ʾһ����ֲ�з���ֲ����ܱ����������������ı仯���ߡ����ͼ�ش�
 |
��1��aͼ�мס������������������ij����ֱ�Ϊ �� ������ ��
��2��aͼ�����У�B�� ������岿λ�������ģ��ҹ����У�A�����÷����ڸù��̵ĵ� �Ρ�
?����3��bͼ�з���ֲ���������ͷŵ�O2���ͺ����������ĵ�O2����ȵĵ��� ���÷���ֲ�꾭��һ��ҹ���Ƿ�������л�� ���� �������� ��
?����.Ⱦ�϶��ȵ���ƣ�DCPIP��������̬������ɫ������ԭʱ�����ַۺ�ɫ����ɫ�����������ʵ�Ҷ����ƽ���ֳɼס���2�ݣ��ֱ�������������0.35mol��L NaCl��Һ��0.035mol��L NaCl��Һ��ҡ���Ƴ���Һ��Һ����Һ����������ʵ�飺
 ��ȡ�ɾ����Թ�4֧�����1��2��3��4�ţ��ںڰ�������ͼ��ʾ�ֱ���������������Һ���Լ���
��ȡ�ɾ����Թ�4֧�����1��2��3��4�ţ��ںڰ�������ͼ��ʾ�ֱ���������������Һ���Լ���
�ڽ���֧�Թ����ڹ���ǿ����ͬ�����˵Ĺ�Դ�£�����ÿ֧�Թ��м���2������ɫ�Ķ��ȵ������Һ��ҡ�ȣ��۲졣
��ʵ���������1���Թܲ������ݣ���Һ������ɫ��ɷۺ�ɫ�����ճ���ɫ��������֧�Թ������ݣ���Һ��������ɫ���ش����⣺?����ʵ������������
��1���Թ������е�����ɷ��� ��
��2���Թ�û�����������ԭ���� ��
��1���Թ�����ɫ��ʧ��ԭ���� ������������������������������������
 ��
��