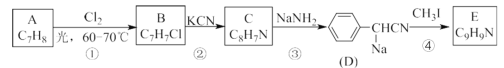
ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩ“―÷ΣΧΰAΡή ΙδεΥ°Ά …ΪΘ§Τδ≤ζΝΩΩ…“‘”Οά¥ΚβΝΩ“ΜΗωΙζΦ“ ·”ΆΜ·ΙΛΖΔ’ΙΥ°ΤΫΓΘAΓΔBΓΔCΓΔDΓΔEΓΔFΓΔGΉΣΜ·ΙΊœΒ»γœ¬Θ®“‘œ¬±δΜ·÷–Θ§”––©Ζ¥”ΠΧθΦΰΦΑ≤ζΈοΈ¥±ξΟςΘ©ΓΘΤδ÷–G «Χλ»Μ”–ΜζΗΏΖ÷Ή”Μ·ΚœΈοΘ§CΓΔFΡήΖΔ…ζ“χΨΒΖ¥”ΠΘ§EΖ÷Ή”ΨΏ”–≈®”τΒΡΙϊœψΈΕΘ§ΤδœύΕ‘Ζ÷Ή”÷ ΝΩΈΣ88ΓΘ

Θ®1Θ©–¥≥ωAΖ÷Ή”ΒΡΒγΉ” ΫΘΚ__ΘΜGΒΡΖ÷Ή” ΫΘΚ__ΓΘ
Θ®2Θ©–¥≥ωΖ÷Ή” Ϋ”κBœύΆ§Θ§ΒΪ≤ΜΡή”κΫπ τΡΤΖ¥”ΠΒΡΈο÷ ΒΡΫαΙΙΦρ Ϋ___ΓΘ
Θ®3Θ©–¥≥ωB+DΓζEΒΡΜ·―ßΖΫ≥Χ ΫΘΚ__ΓΘ
Θ®4Θ©ΙΛ“Β…œ÷Τ±ΗΈο÷ EΒΡΖΫΖ®”–Εύ÷÷ΓΘΤδ÷–A”κD“‘Έο÷ ΒΡΝΩ±»1ΘΚ1Ζ¥”Π…ζ≥…EΘ§«κ≈–ΕœΤδΖ¥”Πάύ–ΆΈΣ__ΘΜΈο÷ CΓΔF__Θ®ΧνΓΑ «Γ±ΜρΓΑ≤Μ «Γ±Θ©Ά§“ΜάύΈο÷ ΓΘ
Θ®5Θ©EΒΡΆ§Ζ÷“λΙΙΧε÷–Θ§Ρή”κNaΖ¥”ΠΘ§”÷Ρή”κNa2CO3»ή“ΚΖ¥”ΠΒΡΈο÷ «__Θ®”ΟΫαΙΙΦρ Ϋ ι–¥“Μ÷÷Φ¥Ω…Θ©
Θ®6Θ©Έο÷ XΩ…”…»γΆΦΉΑ÷Ο÷Τ±ΗΓΘ

ΔΌ»τZ «“Μ÷÷Β≠ΜΤ…ΪΙΧΧεΘ§‘ρΉΕ–ΈΤΩ÷–ΖΔ…ζΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ Ϋ «___ΓΘ
ΔΎ»τZ «“Μ÷÷ΚΎ…ΪΖέΡ©Θ§‘ρΈο÷ Y «__Θ§ZΒΡΉς”Ο «__ΓΘ
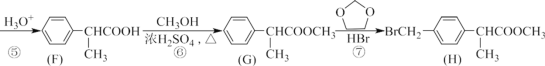
ΓΨ¥πΑΗΓΩ![]() (C6H10O5)n CH3OCH3 CH3COOH+HOCH2CH3
(C6H10O5)n CH3OCH3 CH3COOH+HOCH2CH3![]() CH3COOCH2CH3+H2O Φ”≥…Ζ¥”Π ≤Μ « CH3CH2CH2COOHΜρ
CH3COOCH2CH3+H2O Φ”≥…Ζ¥”Π ≤Μ « CH3CH2CH2COOHΜρ![]() 2Na2O2+2H2O=4NaOH+O2 H2O2Θ®ΥΪ―θΥ°Θ© ¥ΏΜ·Ής”Ο
2Na2O2+2H2O=4NaOH+O2 H2O2Θ®ΥΪ―θΥ°Θ© ¥ΏΜ·Ής”Ο
ΓΨΫβΈωΓΩ
“―÷ΣΧΰAΡή ΙδεΥ°Ά …ΪΘ§Τδ≤ζΝΩΩ…“‘”Οά¥ΚβΝΩ“ΜΗωΙζΦ“ ·”ΆΜ·ΙΛΖΔ’ΙΥ°ΤΫΘ§‘ρAΈΣ““œ©Θ§G «Χλ»Μ”–ΜζΗΏΖ÷Ή”Μ·ΚœΈοΘ§‘ΎΥα–‘ΧθΦΰœ¬Υ°ΫβΒΟFΘ§FΩ…ΖΔ…ζ“χΨΒΖ¥”ΠΘ§GΈΣœΥΈ§ΥΊΜρΒμΖέΘ§FΈΣΤœΧ―Χ«Θ§EΖ÷Ή”ΨΏ”–≈®”τΒΡΙϊœψΈΕΘ§ΤδœύΕ‘Ζ÷Ή”÷ ΝΩΈΣ88Θ§‘ρE τ”ΎθΞΘ§B”κXΖ¥”Π…ζ≥…CΘ§C‘Ό”κXΖ¥”ΠΒΟDΘ§BΓΔDΖ¥”Π…ζ≥…EΘ§CΡήΖΔ…ζ“χΨΒΖ¥”ΠΘ§““œ©Ω…“‘…ζ≥…BΘ§”…¥ΥΩ…ΆΤ÷ΣBΈΣ““¥ΦΘ§CΈΣ““»©Θ§DΈΣ““ΥαΘ§EΈΣ““Υα““θΞΘ§ΤδœύΕ‘Ζ÷Ή”÷ ΝΩΈΣ88Θ§XΈΣ―θΤχΘ§Ψί¥ΥΫβ¥πΓΘ
Θ®1Θ©AΈΣ““œ©Θ§ΧΦ‘≠Ή”÷°Φδ–Έ≥…ΧΦΧΦΥΪΦϋΘ§ΫαΙΙΦρ Ϋ «CH2=CH2Θ§ΤδΒγΉ” ΫΈΣΘΚ![]() Θ§GΈΣœΥΈ§ΥΊΜρΒμΖέΘ§ΤδΖ÷Ή” ΫΈΣ(C6H10O5)nΘΜ¥πΑΗΈΣ
Θ§GΈΣœΥΈ§ΥΊΜρΒμΖέΘ§ΤδΖ÷Ή” ΫΈΣ(C6H10O5)nΘΜ¥πΑΗΈΣ![]() Θ§(C6H10O5)nΓΘ
Θ§(C6H10O5)nΓΘ
Θ®2Θ©BΈΣ““¥ΦΘ§ΤδΫαΙΙΦρ ΫΈΣCH3CH2OHΘ§ΥϋΒΡΆ§Ζ÷“λΙΙΧε≤ΜΡή”κΫπ τΡΤΖ¥”ΠΒΡΈο÷ ΈΣΟ―άύΘ§ΤδΫαΙΙΦρ ΫΈΣCH3OCH3ΘΜ¥πΑΗΈΣCH3OCH3ΓΘ
Θ®3Θ©BΈΣ““¥ΦΘ§DΈΣ““ΥαΘ§Εΰ’ΏΖΔ…ζθΞΜ·Ζ¥”Π…ζ≥…““Υα““θΞΘ§Μ·―ßΖΫ≥Χ ΫΈΣCH3COOH+HOCH2CH3![]() CH3COOCH2CH3+H2OΘΜ¥πΑΗΈΣCH3COOH+HOCH2CH3
CH3COOCH2CH3+H2OΘΜ¥πΑΗΈΣCH3COOH+HOCH2CH3![]() CH3COOCH2CH3+H2OΓΘ
CH3COOCH2CH3+H2OΓΘ
Θ®4Θ©AΈΣ““œ©Θ§DΈΣ““ΥαΘ§A”κD“‘Έο÷ ΒΡΝΩ±»1ΘΚ1Ζ¥”Π…ζ≥…EΘ§ΤδΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣCH2=CH2+CH3COOH![]() CH3COOCH2CH3Θ§ τ”ΎΦ”≥…Ζ¥”ΠΘ§C «““»©Θ§ τ”Ύ»©άύΘ§F «ΤœΧ―Χ«Θ§ τ”ΎΧ«άύΘ§Εΰ’Ώ≤Μ «Ά§“ΜάύΈο÷ ΘΜ¥πΑΗΈΣΦ”≥…Θ§≤Μ «ΓΘ
CH3COOCH2CH3Θ§ τ”ΎΦ”≥…Ζ¥”ΠΘ§C «““»©Θ§ τ”Ύ»©άύΘ§F «ΤœΧ―Χ«Θ§ τ”ΎΧ«άύΘ§Εΰ’Ώ≤Μ «Ά§“ΜάύΈο÷ ΘΜ¥πΑΗΈΣΦ”≥…Θ§≤Μ «ΓΘ
Θ®5Θ©E «““Υα““θΞΘ§ΥϋΒΡΆ§Ζ÷“λΙΙΧε÷–Ρή”κNaΖ¥”ΠΘ§”÷Ρή”κNa2CO3»ή“ΚΖ¥”ΠΒΡΈο÷ «τ»ΥαΘ§ΤδΫαΙΙΦρ ΫΈΣΘΚCH3CH2CH2COOHΜρ![]() ΘΜ¥πΑΗΈΣCH3CH2CH2COOHΜρ
ΘΜ¥πΑΗΈΣCH3CH2CH2COOHΜρ![]() ΓΘ
ΓΘ
Θ®6Θ©XΈΣ―θΤχΘ§ΔΌ»τZ «“Μ÷÷Β≠ΜΤ…ΪΙΧΧεΘ§‘ρZΈΣNa2O2Θ§‘ρΉΕ–ΈΤΩ÷–ΖΔ…ζΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ Ϋ «2Na2O2+2H2O=4NaOH+O2ΓϋΘΜ¥πΑΗΈΣ2Na2O2+2H2O=4NaOH+O2ΓϋΓΘ
ΔΎ»τZ «“Μ÷÷ΚΎ…ΪΖέΡ©Θ§‘ρ «MnO2Θ§‘ρΈο÷ Y «H2O2Θ§ZΒΡΉς”Ο «¥ΏΜ·Ής”ΟΘ§ΤδΜ·―ßΖΫ≥Χ ΫΈΣ2H2O2 ![]() 2H2O+O2ΓϋΘΜ¥πΑΗΈΣH2O2Θ§¥ΏΜ·Ής”ΟΓΘ
2H2O+O2ΓϋΘΜ¥πΑΗΈΣH2O2Θ§¥ΏΜ·Ής”ΟΓΘ