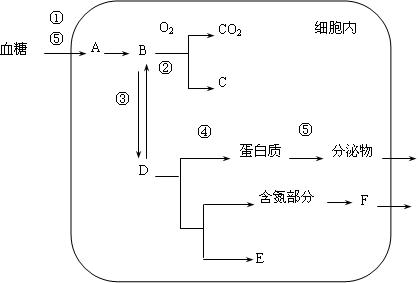
��Ŀ����
��7�֣���ͼ��ʾ�����л�����ʵ�ת���Ѫ�ǵ��ڵ�;�������Т�~�ݱ�ʾ�������̣���~���ʾ����;����A��B�ֱ����������ij�����١����ͼ�ش��������⣺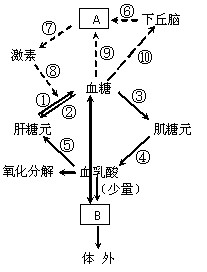
��1��Ѫ�ǵ��ȶ�������Ľ���������Ҫ���á�Ѫ�ǵ���Ҫ������ ����;���������ڢ��������̣���Ѫ�ǵ��ڵ���Ҫ;���� ��������Ű�����;��˳���ʾ����ͼ�в���Ѫ�ǵ��ڵ����ٳ������������ �������ƣ���
��2����Ѫ��Ũ�����ߣ�A���� ����֯ϸ�������
��ϣ��ı�ϸ���Ĵ�л���ٽ���֯ϸ�� ���Ӷ���
��Ѫ��Ũ�ȡ�
��3�������˶�����е���ʹ�Ǽ���Ԫ�ֽ���������ڼ�����
֯�л��۵Ľ������ʹ�ĸо�����λ�� ��

����

�������������е���Ҫ����֮һ����Щ�˺���һ��ƾ����죬���dz�Ϊ�������ˡ������˺��˺ܶ�ƣ���ɫȴû�ж��ٸı䣬���dz�Ϊ�������ˡ�����ͼ��ʾ�Ҵ����������Ĵ�л;�������ͼ�������ش��������⣺

��1���������ˡ�������ֻ��ADH�����ƺ�ѪҺ�� ������Խϸߣ�ëϸѪ�����Ŷ��������졣
��2����ij��������ȩ����ø�����ڽ���������һ��ĸ���ϵ�G��A�����������һ������������û�����������2�κɵõ� ��ͻ������ȩ����ø����
��3����ij��������ͳ�Ʒ�����Ⱥ��ȱ��ADH�ĸ�����81%����һ�Է������ڶ�����ADH�������ӵĸ�������ȱ��ADH����Է�������һ�����ܺϳ�ADH���ӵĸ����� ��
��4��������Ƶķ�������13�����ۺ��������ĸ��ʻ�����13�����ۺ�����һ��Ⱦɫ���쳣�Ŵ�����ҽԺ����Ⱦɫ���ϵ�һ�ζ̴����ظ�������Ϊ�Ŵ����(��+����ʾ�иñ�ǣ���-����ʾ��)���Ըò����п�����ϡ�����ϳ�һ��13�����ۺ�������(���Ϊ��+ - -��)���丸��Ϊ��+ +����ĸ��Ϊ��+ -������û����γ���˫���� �йأ���Ϊ�����γ���ֳϸ�������м������ѵ� ���쳣��
��5����ͼΪϸ���������Ĺ��̣���ͼ�ش�

a.�ܹ����Ŵ���Ϣ��ϸ���˴�����ϸ���ʵ��� (������)������a���������ø�� ��
b.ͼ�к�����̼�ǵ������� (����)��ͼ�Т������صİ������� ��
(�����ӣ�AUG�������ᡢGCU�������ᡢAAG�������ᡢUUC����������)
c. �о�����ijһ�����������ﴦ������������ϸ�����õ�������±���
|
ϸ�������ʺ�����ֵ |
����ǰ |
������ |
|
DNA�sRNA�s������ |
1�s3.1�s11 |
1�s5.4�s21.7 |
�ݴ˷�������ҩ��������ϸ���ķ��ӻ����ǣ�ͨ�� �ٽ�����ı��