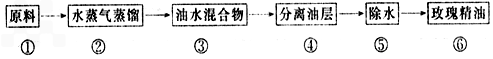
��Ŀ����
(1)��ĸ�����������ѾƲ���ȱ�ٵ�������Ҫ�������Ұ����ĸ����Ҫ��_______���������ý�ĸ�������������Ѿ�Ҫ���Ƶ��¶�Ϊ_________��
(2)�±�Ϊij�������������䷽����ش�
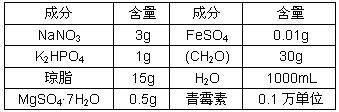
(3)�ͷ�ֳ�����У��������ijɷ���Ⱥ���Ҫ����������Ũ�ȵ������ص���ʹ�ÿ��յ��Թ��� _____ ������ ______�������ʱ�ɴٽ�ѿ����ֳ����Ҫ�����Թ������������ʹ������֯����������������ʹ�õ��������ڼ��� _____ (�����ᡢ2��4-D)
(2)CH2O ѡ�� ����pH
(3)���� ϸ�������� 2��4-D

��08���վ���ѡ����
������A��B���⣬����ѡһ���ڴ��ָ��λ�����𣬲�����ѡ��Ŀ��Ӧ��ĸ��ķ���Ϳ��Ϳ�ڡ�
A�⡣Ϊ̽��ϴ�·ۼ�ø���ϴ��Ч������һ����øϴ�·۷ֳ�3�ȷݣ�����3��ʵ�顣�ס�������ϴ�·��м���1�ֻ�2��ø�����鲻��ø���ڲ�ͬ�¶�����ϴͬ�ֻ��˲��ϵ�2�����գ�����ʵ����������ͬ���±�Ϊʵ���¼����ش��������⡣
ˮ��/�� | 10 | 20 | 30 | 40 | 50 | ||||||||||
��� | �� | �� | �� | �� | �� | �� | �� | �� | �� | �� | �� | �� | �� | �� | �� |
���Ѫ��ʱ��/min | 67 | 66 | 88 | 52 | 51 | 83 | 36 | 34 | 77 | 11 | 12 | 68 | 9 | 11 | 67 |
�������ʱ��/min | 93 | 78 | 95 | 87 | 63 | 91 | 82 | 46 | 85 | 75 | 27 | 77 | 69 | 8 | 68 |
��1�����ϴ�·�ȥ�������ķ�����________��������ϴ�·��м�����________��������ϴ�·��м�����_________��
��2���ס�����ϴ��Ч���IJ��죬˵��ø�����þ���_________��
��3������ס��Һͱ�3�����ˮ��Ϊ80��ʱϴ��ͬһ�����գ���Ƚ���3��ϴ��Ч��֮��IJ��첢˵�����ɡ� ��
��4����øϴ�·��е�ø������Ļ�ѧ���ʰ����ģ���ˮ�������ܿ��ܽ⣬�ͷų�����øѸ�ٷ��Ӵ����á���˵�����Ƿ�������ø�Ĺ̶��������������ɡ�
ѡ���⣨��ѡһ�������ⶼ�𣬰���һ�����
ѡ��A�������е���Ҫ�ɷ��ǵ����ʣ��ڼ�����Һ�У���������CuSO4��Ӧ�ܲ�����ɫ���ʣ����ǵ����ʵ�˫����Ӧ���������һ��Ӧ�������������в���,���һ��ʵ����֤���˵���Һ����ø���յ����ʡ���ѡѡ���ϣ�0��1 g/m�̵�NaOH��Һ��0��01 g/m�̵�CuSO4��Һ��0��05g/m�̵�CuSO4��Һ�������Ե�����Һ��ϡ�͵���Һ����Һ������ˮ���Թܡ��ιܡ���������������ѡ��
ʵ�鲽�裺
�� ȡ2֧�Թܣ����1��2��
�� ��1��2���Թ��зֱ���뵰��Һ����Һ��
��
�� �۲�2֧�Թ�����Һ��ɫ�ı仯��
ʵ����Ԥ�⣺
ʵ�������������ڵ������Ҫ�ɷ���ˮ�͵����ʣ���Һ����Ҫ�ɷ���ˮ����Һ����ø����ˣ�����ʵ��������֤��
ѡ��B: �±���ijʵ��С����֤ø�Ĺ������Եļ���ʵ�飬1��5���Թ���װ�е�����H2O2Һ����ʵ��Ĵ������±���ʾ����ش�
| �Թ���� | ʵ�鴦�� | ||
| ����3%H2O2��mL�� | �¶� | �����Լ� | |
| �Թ�1 | 2 | ���� | 2��3��5%FeCl3Һ |
| �Թ�2 | 2 | ���� | 2��20%������Һ |
| �Թ�3 | 2 | ���� | / |
| �Թ�4 | 2 | 0�� | 2��20%������Һ |
| �Թ�5 | 2 | | |
��2����1��4�ŵĶ���ʵ����,��Ϊ���������3�ţ���1��3��ʵ�����������Ա������� ��
��3����Ҫ֤��ø�Ļ��������ȵ�Ӱ�죬��������5���Թܣ���5���Թܵ�ʵ�鴦�������� ,5���Թ���2���Թܿ���Ϊһ�����ʵ��,�Ӷ�֤��ø�Ļ��������ȵ�Ӱ�졣
ѡ���⣨��ѡһ�������ⶼ�𣬰���һ�����
ѡ��A�������е���Ҫ�ɷ��ǵ����ʣ��ڼ�����Һ�У���������CuSO4��Ӧ�ܲ�����ɫ���ʣ����ǵ����ʵ�˫����Ӧ���������һ��Ӧ�������������в���,���һ��ʵ����֤���˵���Һ����ø���յ����ʡ���ѡѡ���ϣ�0��1 g/m�̵�NaOH��Һ��0��01 g/m�̵�CuSO4��Һ��0��05g/m�̵�CuSO4��Һ�������Ե�����Һ��ϡ�͵���Һ����Һ������ˮ���Թܡ��ιܡ���������������ѡ��
ʵ�鲽�裺
�� ȡ2֧�Թܣ����1��2��
�� ��1��2���Թ��зֱ���뵰��Һ����Һ��
��
�� �۲�2֧�Թ�����Һ��ɫ�ı仯��
ʵ����Ԥ�⣺
ʵ�������������ڵ������Ҫ�ɷ���ˮ�͵����ʣ���Һ����Ҫ�ɷ���ˮ����Һ����ø����ˣ�����ʵ��������֤��
ѡ��B: �±���ijʵ��С����֤ø�Ĺ������Եļ���ʵ�飬1��5���Թ���װ�е�����H2O2Һ����ʵ��Ĵ������±���ʾ����ش�
|
�Թ���� |
ʵ�鴦�� |
||
|
����3%H2O2 ��mL�� |
�¶� |
�����Լ� |
|
|
�Թ�1 |
2 |
���� |
2��3��5%FeCl3Һ |
|
�Թ�2 |
2 |
���� |
2��20%������Һ |
|
�Թ�3 |
2 |
���� |
/ |
|
�Թ�4 |
2 |
0�� |
2��20%������Һ |
|
�Թ�5 |
2 |
|
|
��1����Ҫ��֤����������и�Ч�ԣ���ѡ�õ�ʵ�������
��2����1��4�ŵĶ���ʵ����,��Ϊ���������3�ţ���1��3��ʵ�����������Ա������� ��
��3����Ҫ֤��ø�Ļ��������ȵ�Ӱ�죬��������5���Թܣ���5���Թܵ�ʵ�鴦�������� ,5���Թ���2���Թܿ���Ϊһ�����ʵ��,�Ӷ�֤��ø�Ļ��������ȵ�Ӱ�졣