题目内容
【题目】下图是体育运动对学习记忆的促进作用与蛋白质类神经营养因子(BDNF)关系的部分图解。请据图回答问题:
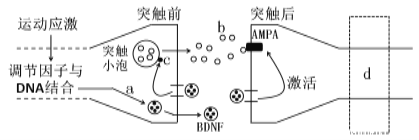
(1)突触小泡中的b物质是__________,该物质通过__________方式进入突触间隙。
(2)请画出b物质与AMPA结合后兴奋传导至d处时,细胞膜内外电荷的分布情况________________。
(3)据图可知,BDNF具有____________和激活突触后膜上相应受体的作用,从而促进兴奋在突触处的传递。若向大鼠脑室内注射抗BDNF的抗体,将导致突触间隙内b物质的含量____________(填“增加”/“不变”/“减少”)。
(4)图中c是突触蛋白,它在海马区的密度和分布可间接反映突触的数量和分布情况。有实验表明,水迷宫训练后大鼠海马区突触蛋白表达明显增强,大鼠学习记忆受损后突触蛋白表达水平下降。由此推测,长期记忆可能与____________的建立有关。
【答案】(1)神经递质 胞吐(外排) (2)见右图 
(3)促进神经递质的释放 减少 (4)(新)突触
【解析】(1)兴奋经突触传递时,突触小泡向突触前膜移动,以胞吐的方式向突触间隙释放突触小泡中的神经递质,体现了细胞膜具有流动性。(2)b与AMPA结合后兴奋传导至d处时,细胞膜内外电荷的分布情况,如图(见参考答案)。(3)据图可知,BDNF具有促进神经递质的释放和激活突触后膜上相应受体的作用,从而促进兴奋在突触处的传递。若向大鼠脑室内注射抗BDNF的抗体,则会抑制神经递质的释放,将导致突触间隙内b的数量减少。(4)根据突触蛋白c能在海马区的密度和分布可间接反映突触的数量和分布情况,说明长期记忆可能与新突触的建立有密切关系。

练习册系列答案
 优生乐园系列答案
优生乐园系列答案 新编小学单元自测题系列答案
新编小学单元自测题系列答案
相关题目