��Ŀ����
����Ŀ����-2-��ϩȩ(D)��һ����Ҫ�ĺϳ����ϣ����кϳ�·�����Ʊ�D�ķ���֮һ�����ݸúϳ�·�ش��������⣺

��֪��
![]()
(1)A��������__________��B�����й���ԭ����Ŀ���Ϊ__________��C�������뻷���������������У���ͬ��ѧ��������ԭ�ӹ���_____�֡�
(2)D�к��������ŵ�������__________��д������ù����ŵĻ�ѧ��Ӧ����ʽ___________________________
(3)EΪ�л����˴Ź���������ʾ�����ַ壬�������Ϊ_________���ܷ����ķ�Ӧ��__________������ĸ��
a.�ۺϷ�Ӧb.�ӳɷ�Ӧc.��ȥ��Ӧd.ȡ����Ӧ
(4)B��ͬ���칹��F��B����ȫ��ͬ�Ĺ����ţ�д��F���п��ܵĽṹ��ʽ________________________________��������˳���칹��
(5)��DΪ��Ҫԭ���Ʊ���ȩ(Ŀ�껯����)���ڷ����н��ϳ�·�ߵĺ�벿�ֲ���������
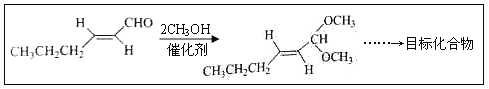
___________________________________________________________��
(6)����(5)�ĺϳ�·���е�һ����Ӧ��Ŀ����__________��
���𰸡�����ȩ��ȩ 9 8 ȩ�� ![]() +2Ag(NH3)2OH
+2Ag(NH3)2OH![]()
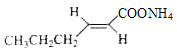 +2Ag��+3NH3+H2O��
+2Ag��+3NH3+H2O��![]() +2Cu(OH)2+NaOH
+2Cu(OH)2+NaOH![]()
![]() +Cu2O��+3H2O 3:2:1 cd CH2=CHCH2OCH3��
+Cu2O��+3H2O 3:2:1 cd CH2=CHCH2OCH3��![]() ��
��![]() ��
��![]()
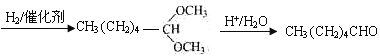 ����ȩ��(������������)
����ȩ��(������������)
��������
��1������ϩ��ƽ��ṹ����������������ṹ�жϹ���ԭ������
��2������ȩ��������������Һ������������ͭ����Һ��
��3�������֪������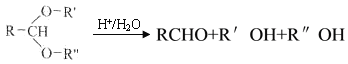 ������
������
��4��BΪCH2=CHOC2H5������̼̼˫�����Ѽ�����̼���칹������λ���칹������
��5��������Ϣ�ͼ�ȩ�Ľṹ��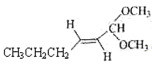 ������Ҫ��̼̼˫��ת��Ϊ������Ȼ�������������·�Ӧ���ɣ�
������Ҫ��̼̼˫��ת��Ϊ������Ȼ�������������·�Ӧ���ɣ�
��6��ȩ��Ҳ�ܹ��������ӳɡ�
��1����������ͼ��AΪCH3CH2CH2CHO������Ϊ��ȩ��BΪCH2=CHOC2H5�������й���ԭ����Ŀ���Ϊ9��(��ͼ��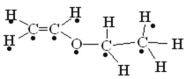 )��C�������뻷���������������У�8��̼ԭ���ϵ���ԭ�ӻ�ѧ����������ͬ������8�֣�
)��C�������뻷���������������У�8��̼ԭ���ϵ���ԭ�ӻ�ѧ����������ͬ������8�֣�
��2��DΪ![]() �����к�����������ȩ��������ȩ��������������Һ������������ͭ����Һ������ȩ���Ļ�ѧ��Ӧ����ʽΪ
�����к�����������ȩ��������ȩ��������������Һ������������ͭ����Һ������ȩ���Ļ�ѧ��Ӧ����ʽΪ![]() +2Ag(NH3)2OH
+2Ag(NH3)2OH![]()
 +2Ag��+3NH3+H2O��
+2Ag��+3NH3+H2O��![]() +2Cu(OH)2+NaOH
+2Cu(OH)2+NaOH![]()
![]() +Cu2O��+3H
+Cu2O��+3H
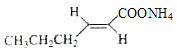
(3)��������ͼ�������Ϣ��C�����������·�Ӧ����![]() ��CH3CH2CH2CHO��CH3CH2OH�Լ�ˮ��EΪ�л����˴Ź���������ʾ�����ַ壬���EΪCH3CH2OH���������Ϊ3:2:1��CH3CH2OH���ڴ����ܷ����ķ�Ӧ����ȥ��Ӧ��ȡ����Ӧ����ѡcd��
��CH3CH2CH2CHO��CH3CH2OH�Լ�ˮ��EΪ�л����˴Ź���������ʾ�����ַ壬���EΪCH3CH2OH���������Ϊ3:2:1��CH3CH2OH���ڴ����ܷ����ķ�Ӧ����ȥ��Ӧ��ȡ����Ӧ����ѡcd��
(4) BΪCH2=CHOC2H5������̼̼˫�����Ѽ���B��ͬ���칹��F��B����ȫ��ͬ�Ĺ����ţ� F���ܵĽṹ�У�CH2=CHCH2OCH3��![]() ��
��![]() ��
��![]() ��
��
��5��DΪ![]() ����ȩ�Ľṹ��ʽΪCH3CH2CH2CH2CH2CHO��������Ϣ�ͼ�ȩ�Ľṹ��
����ȩ�Ľṹ��ʽΪCH3CH2CH2CH2CH2CHO��������Ϣ�ͼ�ȩ�Ľṹ�� ������Ҫ��̼̼˫��ת��Ϊ������Ȼ�������������·�Ӧ���ɣ�
������Ҫ��̼̼˫��ת��Ϊ������Ȼ�������������·�Ӧ���ɣ�
��6��ȩ��Ҳ�ܹ��������ӳɣ���5���кϳ�·���е�һ����Ӧ��Ŀ���DZ���ȩ����

 ���Ͱ�ͨ�������Сѧ��ʱͬ�����ϵ�д�
���Ͱ�ͨ�������Сѧ��ʱͬ�����ϵ�д� ���Ͱ�ͨ������ϵ�д�
���Ͱ�ͨ������ϵ�д� �ٷ�ѧ����ҵ��������ϵ�д�
�ٷ�ѧ����ҵ��������ϵ�д�