��Ŀ����
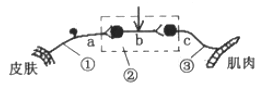
����Ŀ����ͼ��������������ͼ�⣬����ͼ�ش����⣺ 
��1��д��1��2��3���������������ƣ�1�� �� 2�� �� 3�� ��
��2��д��4��5��6�������������Ķ��٣�����������������������������4�� �� 5�� �� 6�� ��
��3��������������Ҫ������ �� ����ó����ĺ�����Ӧ���� ��
��4�����O2��Ӧ���㣬��������C6H12O6�ķֽ������ �� ��Ӧ������ ��
���𰸡�
��1����ͪ�H2O��CO2
��2������ATP����������������ATP����������������ATP����������
��3�������壻��ͪ��
��4�����ϸ���ʻ���
���������⣺��1��������ͼ��֪��1�DZ�ͪ�ᣬ2�Dz���ˮ��3�Dz��������̼����2��4������������һ���ͷŵ�������5�����������ڶ����ͷŵ�������6���������������ͷŵ����������������ĵ�һ���ڶ����ͷŵ������٣��������ͷŵ������࣮��3�����������ķ�Ӧ����Ϊϸ���ʻ��ʺ������壬��Ҫ����Ϊ�����壬��ͪ�����������������������ĵڶ������Σ���4��������Ӧ���㣬ϸ������������������������C6H12O6�ķֽ���������ᣬ��Ӧ������ϸ���ʻ��ʣ�
�ʴ�ӦΪ����1����ͪ�� H2O CO2��2������ ATP���������� ���� ATP���������� ����ATP������������3�������� ��ͪ�ᣨ4������ ϸ���ʻ���
�����㾫����������Ҫ���������������������κ����������ĸ�������̵����֪ʶ�㣬��Ҫ�������������������εij�����ϸ���ʻ��ʣ���������ʣ���������Ĥ��3���εĸ�����ѧ��Ӧ���ɲ�ͬ��ø�����ģ�һ����ָϸ���������������£�ͨ��ø�Ĵ����ã��������ǵ��л���ֽ�Ϊ��������������ƾ���CO2�����ᣩ��ͬʱ�ͷų����������Ĺ��̲�����ȷ�����⣮