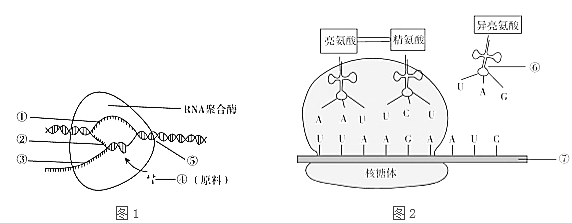
��Ŀ����
����Ŀ����۹�ʵ��ơ����㣬����ά���� C�����߰�ȳɷ֣����нϸߵ�ʳ�ü�ֵ����Ƥɫ�ضԴ˾�����һ���� �������á���ش������й����⣺
��1�����ý����Ȼ���Ͳ������ƣ����õ�������Ҫ��_____�����������¶���Ҫ������_____�淶 Χ�ڡ�
��2�����Ƥ�������˲���_____����ȡ��Ƥ���ͣ��ù����еõ��ĺ�״Һ���ͨ��_____��ȥ�� �еĹ������ʡ�
��3����ʣ�Ľ�۾Ʒ���һ��ʱ�������ԭ����_____�����ᷢ�͵��¶�Ҫ������__________�档
���𰸡���ĸ�� 18��25 ѹե ���� ����������,�����еĴ����������������Ͳ����˴��� 30-35
��������
������������������ǽ�ĸ�������³´�л����Ϊ�������������ͣ�����������ԭ����
��1�������������£���Ӧʽ���£�C6H12O6+6H2O+6O2![]() 6CO2+12H2O+������
6CO2+12H2O+������
��2�������������£���Ӧʽ���£� C6H12O6![]() 2C2H5OH+2CO2+������
2C2H5OH+2CO2+������
��1�����Ʒ������õ�������Ҫ�ǽ�ĸ�������������¶���Ҫ������18��25����Χ�ڡ�
��2�����Ƥ�������˲���ѹե����ȡ��Ƥ���ͣ��ù����еõ��ĺ�״Һ���ͨ�����˳�ȥ���еĹ������ʡ�
��3���������Ĺ��Ʒ���һ��ʱ�����ʱ�������ζ����ԭ���������ܱղ��ϣ��������������������������ת��ɴ��ᣨ���ᣩ�����ᷢ�͵��¶�Ҫ������30��35����Χ�ڡ�

 ��У����ϵ�д�
��У����ϵ�д�