��Ŀ����
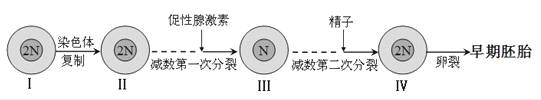
����Ŀ����ͼ��ʾ��������������Ũ�����������õĹ�ϵ����ͼ��ʾ��һ����������ˮƽ���ã�����һ��ʱ��������״������ͼ��ʾ������ѿ�ʡ���ش�

��1���ɼ�ͼ��֪�����ڲ�ͬ��������˵�����شٽ�����������Ũ�Ȳ�ͬ������ͬһ������˵�������ص�������Ũ�ȵĹ�ϵ��________________________________________________��
��2����ͼ��a��������Ũ�ȴ���________________________mol/L��
��3��������������������������������ص����þ���________________���ص㡣
��4��Ϊ��֤�ڵ�������£���ͼ������ѿ�ʼ�˲����������صĺ������䷢����A �ζ�����B�Σ�ijͬѧ���������ʵ�鲽�裬���������������й�ʵ����̣�
I��ʵ����ϼ��þߣ�������ѿ�ʡ�һ���Ӳֽ�С�����ĸƬ����Դ�ȡ�
����ͼ��ʾ���Ǹ�ʵ����̡�

��ʵ����������
װ�â�����ѿ��___________������������_________________________________________��
װ�âڢ�����ѿ��_________������������_________________________________________��
���𰸡� ��һ��Ũ�ȷ�Χ�ڴٽ������������⡪��Χ���������� 10-8 ������ ֱ�� ��ѿ�ʵ�B��û�з��������صĺ������� �����Դ ��ѿ��A�η����������صĺ�������
�����������������������ͼ��ͼʾ��ʾ������Ũ�ȶ�ֲ�����ѿ������Ӱ�죬��ͬ���ٶ������ص����г̶Ȳ�ͬ���������С�ѿ��Σ������شٽ�����ѿ������������Ũ�ȷֱ���10-10mol/L��10-8mol/L��������ͼ�������ܵ��������ص�Ӱ�죬a���������Ũ�ȸ���b�㡣����֤�ڵ���������£���ͼ������ѿ�ʼ�˲����������صĺ������䷢����A�ζ�����B�Ρ����ɶ�������ѿ�ʽ������ִ��������A�Σ����B�Σ�������κβ�λ�������ա�
��1����ͼ������֪�����ڲ�ͬ��������˵�����شٽ�����������Ũ�Ȳ�ͬ������ͬһ������˵����������һ��Ũ�ȷ�Χ�ڴٽ�������������һ��Χ������������
��2����3��ͼ�и���������Ũ��������ǿ�����ز�a��������Ũ�ȸߣ����� 10-8mol/L�������˸���������Զ�ز�������Ũ�ȵͣ��ٽ��˸�����������˸�������������������������õ������ԡ�
��4����ʵ���Ŀ��������֤�ڵ���������£���ͼ������ѿ�ʼ�˲����������صĺ������䷢����A�ζ�����B�������ɶ�������ѿ�ʽ������ִ��������A�Σ����B�Σ�������κβ�λ����������������ʵ���飺��ѿ�ʵļ������ĸƬ����������������ڼ�˵ĺ������䣬ʹ������ͱ����������طֲ����ȣ�������ѿ��ֱ���������ڢ������Ƕ����飺��ѿ�ʵļ��û����ĸƬ�����ڼ�˷����˺������䣬ʹ�ñ�����������Ũ�ȱ�����Ũ�ȸߣ����Ա���������ıȽϿ�Ӷ�������ѿ���������������

����Ŀ���о���ȡҰ����С��I+I+������̥��ϸ����ת�뺬neor������ù�ؿ��Ի���DNAƬ�Σ�����ͻ��I+�������ͼa�����ٽ�ͻ�����̥��ϸ���ƻ�Ұ����С����̥����������ͻ�����I-�����Ӻ���С��

��1������ԴDNAƬ�ε�����̥��ϸ�������ú�__________������������ϸ������ɸѡ�õ�ͻ�����̥��ϸ����
��2���ô��Ӻ���С����Ұ����С������ӽ�ʵ�飬��ͨ��DNA�����ӽ��������С��Ļ����ͣ������ͼb��

�ټ��С��Ļ����ͣ������__________�������DNA����̽�롣�����ӽ�ʵ�����Ʋ⣬I����Ĺ�������__________�йء�
�ڷ���ϵ��ͼ__________����/���ܣ��ж�I+��I-������ԡ����ԣ�������____________________��
��ֻ��ͻ���������__________������/ĸ����ʱ���Ӻ���С��ű��ֳ������ٻ����ɴ��Ʋ�����____________________��I��������ϸ���в����
����ȡС����ϸ������RNA������Actin������ϸ���Ǽܵ��ף���neor�����RNA̽�룬֮�����RNAøˮ�ⵥ��RNA����̽������ϸ����Ʒ��RNA��ϳ�˫��RNA��øˮ�����������Ӿ����ʱ���������������ڼ�¼ʵ����ʱ�������� �����á�+����ʾ�������������á�-����ʾ�����뽫֧�֢��Ʋ��ʵ���������±�i��ii��iii����
Ұ����С�� | ͻ��������Ը������Ӻ���С�� | ͻ���������ĸ�����Ӻ���С�� | |
Actin�����RNA̽�� | + | + | + |
neor�����RNA̽�� | i__________ | ii__________ | iii__________ |
��3���Ӻ���С����۸��彻�䣬�����ı����ͼ�����Ϊ__________��