��Ŀ����
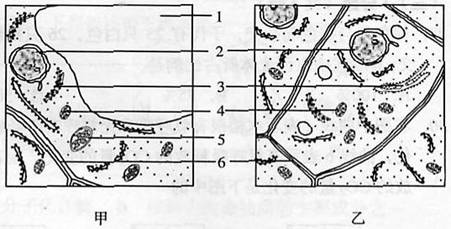
�ס�����ͼ�Ǻ��Ҷ��ϸ������������ϸ���������ṹʾ��ͼ����ش��������⡣
(1)��������ϸ���ֱ�����30��������Һ�У����ܷ����ʱڷ��������ϸ���� ��ͼ�нṹ [ ] ����Ҫ���á�([ ]������)
(2)�ס�����ͼ�о���˫��Ĥ�ṹ��ϸ����������
(3)�Ա��4�ͱ��5��ʾ�ṹ������ȷ���ǣ� ��
| A��������ж�����ˮ | B��ǰ����Ƭ��ṹ���������� |
| C���ڰ������£�������ж�����O2 | D��ǰ�߲�����ATP�����߲���ATP |
�ٲ���ֳ ���ܼ�����ֳ ��ϸ����DNAֻת¼������
�ܳ���Ⱦɫ��ṹ ����mRNA���˿���ϸ������
(1)ͼ�� [1]��Һ�� ��2�������� Ҷ����
(3��A ��4���٢ۢ� �ڢܢ�
�����������������������ϸ�����ڽ�����˿���ѣ���Һ�ݣ��������Ǹ���������ϸ������ͼ�д�Һ�ݣ���Һ��Ĥ��ϸ��Ĥ��ϸ���ʹ��ɵ�ԭ���ʲ���һ���Ĥ��ϸ��Һ�����������Һ��Ũ�Ȳ�ܷ����ʱڷ��롣6��ϸ���ڣ�����Ҫ�ɷ�����ά�ء������塢Ҷ�����Ǿ�˫��Ĥ�ṹ��ϸ������4��5�ֱ����������Ҷ���壬������úͺ������þ��ܲ���ˮ��ATP���ڰ������£�ֻ�������������������������ͼ��Ҷ��ϸ���ѷֻ���������ֳ������ϸ������DNA�����ƣ����е����ʺϳɣ���DNAת¼��mRNA���˿���ϸ���ʣ�ͼ�������ڷ��ѵĸ���������ϸ��������ֳ������Ⱦɫ�壬��mRNA���˿���ϸ�����С�
���㣺 ����ϸ������ͼ���ʶ��
������ ������ֲ���ϸ������������Ҷ���塢��Һ�ݺ�ϸ���ڣ��ߵ�ֲ��û�������塣���Ϸ��ѵ�ֲ��ϸ��û��Ҷ����ʹ�Һ�ݣ�ԭ���ʲ���ָϸ��Ĥ��Һ��Ĥ�Լ�����Ĥ֮���ϸ���ʡ�����ѡ�����ԡ�Ҷ�����ǹ�����õij�������������������������Ҫ������

��11�֣��ס�����ͼ�Ǻ��Ҷ��ϸ������������ϸ�������ṹʾ��ͼ����ش��������⣺?
��1����ʾ����������ϸ������ͼ ����������ϸ���ֱ�����30%������Һ�У����ܷ����ʱڷ��������ϸ����ͼ ��ͼ�нṹ�� �� ����Ҫ���á�
��2��ͼ�б��6��ʾ�ṹ����Ҫ�ɷ��� ��
��3���ס�����ͼ�о���˫��Ĥ�ṹ��ϸ���������� ��
��4���Ա��4�ͱ��5��ʾ�ṹ������ȷ���ǣ� ��
| A��������ж�����ˮ | B��ǰ����Ƭ��ṹ���������� |
| C���ڰ������£�������ж�����O2 | D��ǰ�߲�����ATP�����߲���ATP |
 �������� ���������÷�ʽ����������IJ�����ϸ���е� �� ����ϸ������ֱ�ӵĹ�ϵ��
�������� ���������÷�ʽ����������IJ�����ϸ���е� �� ����ϸ������ֱ�ӵĹ�ϵ��